当前位置:
X-MOL 学术
›
Mol. Syst. Des. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In silico strategy for isoform-selective 5-HT2AR and 5-HT2CR inhibitors
Molecular Systems Design & Engineering ( IF 3.2 ) Pub Date : 2021-1-7 , DOI: 10.1039/d0me00137f Xiaohui Geng 1, 2, 3, 4 , Ying Wang 2, 3, 4, 5 , Huibin Wang 1, 2, 3, 4 , Baichun Hu 2, 3, 4, 6, 7 , Junhao Huang 1, 2, 3, 4 , Yiheng Wu 2, 3, 4, 6, 7 , Jian Wang 2, 3, 4, 6, 7 , Fengjiao Zhang 2, 3, 4, 5
Molecular Systems Design & Engineering ( IF 3.2 ) Pub Date : 2021-1-7 , DOI: 10.1039/d0me00137f Xiaohui Geng 1, 2, 3, 4 , Ying Wang 2, 3, 4, 5 , Huibin Wang 1, 2, 3, 4 , Baichun Hu 2, 3, 4, 6, 7 , Junhao Huang 1, 2, 3, 4 , Yiheng Wu 2, 3, 4, 6, 7 , Jian Wang 2, 3, 4, 6, 7 , Fengjiao Zhang 2, 3, 4, 5
Affiliation
|
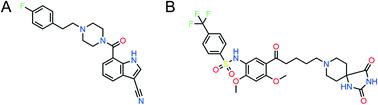
5-HT2AR and 5-HT2CR are widely expressed throughout the brain and have been drawing significant clinical interest due to their involvement in mediating mental disorders. Indeed, 5-HT2AR acts as a biological target for atypical antipsychotics and addiction, and 5-HT2CR also serves as a therapeutic target for depression and addiction. Given that 5-HT2AR and 5-HT2CR provide opposing influences upon DA mesocorticoaccumbens output, plus 5-HT2CR is thought to be involved in appetite that might result in the side effect of weight gain, it makes great sense for drug design to provide the selective mechanisms of 5-HT2AR and 5-HT2CR in order to meet the increasing need for more effective medications. Toward this end, the structure and chemical properties of crucial residues between 5-HT2AR and 5-HT2CR were analyzed based on their individual crystal structures. Moreover, pruvanserin and RS102221, the highly selective antagonists of 5-HT2AR and 5-HT2CR, respectively, were employed to illuminate the selective binding modes through a comprehensive application of in silico methods, including molecular docking, molecular dynamic simulations, MM-GBSA, alanine scanning mutagenesis, DFT technologies, and structure-based pharmacophore modeling. It was found that although 5-HT2A/CR isoforms share high sequence homology in active pockets, they do possess distinctive physiological functions. The key residues that contributed to the selectivity between 5-HT2A/CR are ASP155, LEU229, TRP336, and PHE340 of 5-HT2AR, as well as ASP134, LEU209, and PHE328 of 5-HT2CR. Moreover, in view of the much smaller hydrophobic region of the 5-HT2AR, a ligand with reduced side chain volume would increase its selectivity to 5-HT2AR, and vice versa. In addition, considering that RS102221/5-HT2CR formed more water bridges compared to that of pruvanserin/5-HT2AR, hydrogen bonding interactions should be emphasized in the design of 5-HT2CR selective inhibitors. Collectively, this study provided novel insights into the rational selectivity mechanisms of 5-HT2AR/5-HT2CR inhibitors, which laid important foundations for designing selective inhibitors towards 5-HT2AR and 5-HT2CR.
中文翻译:

选择性异构体5-HT2AR和5-HT2CR抑制剂的计算机策略
5-HT 2A R和5-HT 2C R在整个大脑中广泛表达,并且由于它们参与介导精神障碍而引起了极大的临床兴趣。实际上,5-HT 2A R充当非典型抗精神病药和成瘾的生物学目标,而5-HT 2C R亦充当抑郁和成瘾的治疗目标。鉴于5-HT 2A R和5-HT 2C R对DA中肠球菌的产量有相反的影响,另外5-HT 2C R被认为与食欲有关,可能导致体重增加的副作用,因此对于提供5-HT 2A R和5-HT选择性机制的药物设计图2C中的R,以满足对更有效的药物不断增长的需要。为此,根据5-HT 2A R和5-HT 2C R之间的关键晶体结构,分析了它们的关键结构和化学性质。此外,pruvanserin和RS102221,5-HT的高度选择性的拮抗剂2A R和5-HT 2C R,分别被雇用通过的综合应用以照亮选择性结合模式,在硅片的方法,包括分子对接,分子动力学模拟, MM-GBSA,丙氨酸扫描诱变,DFT技术和基于结构的药效团建模。发现尽管5-HT 2A / CR同工型在活跃的口袋中具有高度的序列同源性,它们确实具有独特的生理功能。关键残基促成5-HT之间的选择性2A / C R 2是ASP155,LEU229,TRP336,和5-HT的PHE340 2A R,以及5-HT的ASP134,LEU209,PHE328和2C R.另外,鉴于5-HT 2A R的疏水区域小得多,侧链体积减小的配体将增加其对5-HT 2A R的选择性,反之亦然。另外,考虑到与pruvanserin / 5-HT 2A相比,RS102221 / 5-HT 2C R形成了更多的水桥R,在5-HT 2C R选择性抑制剂的设计中应强调氢键相互作用。总的来说,这项研究为5-HT 2A R / 5-HT 2C R抑制剂的合理选择性机制提供了新颖的见解,为设计针对5-HT 2A R和5-HT 2C R的选择性抑制剂奠定了重要基础。
更新日期:2021-01-18
中文翻译:

选择性异构体5-HT2AR和5-HT2CR抑制剂的计算机策略
5-HT 2A R和5-HT 2C R在整个大脑中广泛表达,并且由于它们参与介导精神障碍而引起了极大的临床兴趣。实际上,5-HT 2A R充当非典型抗精神病药和成瘾的生物学目标,而5-HT 2C R亦充当抑郁和成瘾的治疗目标。鉴于5-HT 2A R和5-HT 2C R对DA中肠球菌的产量有相反的影响,另外5-HT 2C R被认为与食欲有关,可能导致体重增加的副作用,因此对于提供5-HT 2A R和5-HT选择性机制的药物设计图2C中的R,以满足对更有效的药物不断增长的需要。为此,根据5-HT 2A R和5-HT 2C R之间的关键晶体结构,分析了它们的关键结构和化学性质。此外,pruvanserin和RS102221,5-HT的高度选择性的拮抗剂2A R和5-HT 2C R,分别被雇用通过的综合应用以照亮选择性结合模式,在硅片的方法,包括分子对接,分子动力学模拟, MM-GBSA,丙氨酸扫描诱变,DFT技术和基于结构的药效团建模。发现尽管5-HT 2A / CR同工型在活跃的口袋中具有高度的序列同源性,它们确实具有独特的生理功能。关键残基促成5-HT之间的选择性2A / C R 2是ASP155,LEU229,TRP336,和5-HT的PHE340 2A R,以及5-HT的ASP134,LEU209,PHE328和2C R.另外,鉴于5-HT 2A R的疏水区域小得多,侧链体积减小的配体将增加其对5-HT 2A R的选择性,反之亦然。另外,考虑到与pruvanserin / 5-HT 2A相比,RS102221 / 5-HT 2C R形成了更多的水桥R,在5-HT 2C R选择性抑制剂的设计中应强调氢键相互作用。总的来说,这项研究为5-HT 2A R / 5-HT 2C R抑制剂的合理选择性机制提供了新颖的见解,为设计针对5-HT 2A R和5-HT 2C R的选择性抑制剂奠定了重要基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号