当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Switching between mono and doubly reduced odd alternant hydrocarbon: designing a redox catalyst
Chemical Science ( IF 7.6 ) Pub Date : 2020-12-23 , DOI: 10.1039/d0sc05972b Jasimuddin Ahmed 1 , Paramita Datta 1 , Arpan Das 1 , Stephy Jomy 1, 2 , Swadhin K Mandal 1
Chemical Science ( IF 7.6 ) Pub Date : 2020-12-23 , DOI: 10.1039/d0sc05972b Jasimuddin Ahmed 1 , Paramita Datta 1 , Arpan Das 1 , Stephy Jomy 1, 2 , Swadhin K Mandal 1
Affiliation
|
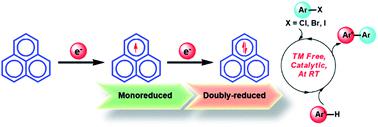
Since the early Hückel molecular orbital (HMO) calculations in 1950, it has been well known that the odd alternant hydrocarbon (OAH), the phenalenyl (PLY) system, can exist in three redox states: closed shell cation (12π e−), mono-reduced open shell neutral radical (13π e−) and doubly reduced closed shell anion (14π e−). Switching from one redox state of PLY to another leads to a slight structural change owing to its low energy of disproportionation making the electron addition or removal process facile. To date, mono-reduced PLY based radicals have been extensively studied. However, the reactivity and application of doubly reduced PLY species have not been explored so far. In this work, we report the synthesis of the doubly reduced PLY species (14π e−) and its application towards the development of redox catalysis via switching with the mono-reduced form (13π e−) for aryl halide activation and functionalization under transition metal free conditions without any external stimuli such as heat, light or cathodic current supply.
中文翻译:

在单还原和双还原的奇数交替烃之间切换:设计氧化还原催化剂
由于休克尔早期分子轨道(HMO)在1950年的计算中,已经公知的是奇数交替烃(OAH)时,非那烯基(PLY)系统中,可以在三个氧化还原状态存在:封闭壳体阳离子(12πë - ),单减小开壳中性自由基(13πë - )和成倍减少封闭壳阴离子(14πë - )。由于PLY的歧化能低,因此从PLY的一种氧化还原状态切换到另一种状态会导致轻微的结构变化,从而使电子添加或去除过程变得容易。迄今为止,已经广泛研究了单还原的基于PLY的自由基。然而,迄今为止还没有研究双还原的PLY物种的反应性和应用。在这项工作中,我们报告了双还原PLY物种(14πe- )和其对氧化还原催化的开发应用程序通过与单-还原形式切换(13πë - )对芳基卤化物的活化和过渡金属的分类的条件下的官能化没有任何外部刺激如热,光或阴极电流供给。
更新日期:2021-01-18
中文翻译:

在单还原和双还原的奇数交替烃之间切换:设计氧化还原催化剂
由于休克尔早期分子轨道(HMO)在1950年的计算中,已经公知的是奇数交替烃(OAH)时,非那烯基(PLY)系统中,可以在三个氧化还原状态存在:封闭壳体阳离子(12πë - ),单减小开壳中性自由基(13πë - )和成倍减少封闭壳阴离子(14πë - )。由于PLY的歧化能低,因此从PLY的一种氧化还原状态切换到另一种状态会导致轻微的结构变化,从而使电子添加或去除过程变得容易。迄今为止,已经广泛研究了单还原的基于PLY的自由基。然而,迄今为止还没有研究双还原的PLY物种的反应性和应用。在这项工作中,我们报告了双还原PLY物种(14πe- )和其对氧化还原催化的开发应用程序通过与单-还原形式切换(13πë - )对芳基卤化物的活化和过渡金属的分类的条件下的官能化没有任何外部刺激如热,光或阴极电流供给。











































 京公网安备 11010802027423号
京公网安备 11010802027423号