当前位置:
X-MOL 学术
›
Can. J. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Optimal conditions determination for hydrodeoxygenation of free fatty acids to obtain green diesel
The Canadian Journal of Chemical Engineering ( IF 1.6 ) Pub Date : 2021-01-17 , DOI: 10.1002/cjce.24035 J.F. Durán‐Pérez 1 , E. Zamora Rodea 1 , A.K. Medina Mendoza 1 , M.M. González‐Brambila 1 , C. Tapia 1 , J.A. Colín‐Luna 1 , J. C. García‐Martínez 2
The Canadian Journal of Chemical Engineering ( IF 1.6 ) Pub Date : 2021-01-17 , DOI: 10.1002/cjce.24035 J.F. Durán‐Pérez 1 , E. Zamora Rodea 1 , A.K. Medina Mendoza 1 , M.M. González‐Brambila 1 , C. Tapia 1 , J.A. Colín‐Luna 1 , J. C. García‐Martínez 2
Affiliation
|
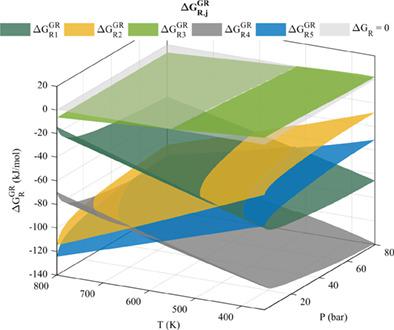
In this work, the production of green diesel involving the hydrodeoxygenation reaction of vegetable oil, such as oleic acid, producing CO or CO2 as byproducts, was studied. This reaction takes place under hydrogen saturation to produce stearic acid; subsequently, heptadecane is produced by the decarbonylation and decarboxylation pathways, while octadecane is obtained by the subsequent hydrodeoxygenation of octadecanal. In the formation of octadecane, water is obtained as a byproduct, while the production of heptadecane leads to CO and CO2 as byproducts of the decarbonylation and decarboxylation reactions. When considering the products and reaction routes in the hydrotreatment of triglycerides and free acids, a mathematical model was found to be reliable in determining the minimization of the Gibbs free energy of reaction over a range of reaction temperatures and pressures. This model followed the methods of Marrero‐Gani, Joback‐Reid, and Chen‐Dinivahi‐Jeng for the estimation of thermodynamic properties of the pure components. The fugacity was calculated by considering the partial fugacity coefficients, and the Peng‐Robinson equation of state and the van der Waals mixing rule were used. The mole fractions at equilibrium were estimated by least squares analysis, using MATLAB, in order to account for non‐idealities in reaction thermodynamic properties and to find the equilibrium composition that minimized the total Gibbs free energy. Likewise, the reaction coordinates of the equilibrium reaction were estimated together with the known values of the composition in order to know the effect of pressure and temperature on the aforementioned reactions.
中文翻译:

游离脂肪酸加氢脱氧以获得绿色柴油的最佳条件确定
在这项工作中,研究了绿色柴油的生产,该绿色柴油涉及植物油(例如油酸)的加氢脱氧反应,产生副产物CO或CO 2。该反应在氢饱和下进行以产生硬脂酸;随后,通过脱羰和脱羧途径生成庚烷,而随后通过十八烷的加氢脱氧获得十八烷。在十八烷的形成过程中,会获得水作为副产物,而十七烷的产生会导致CO和CO 2的产生。作为脱羰和脱羧反应的副产物。当考虑甘油三酸酯和游离酸的加氢处理中的产物和反应路线时,发现数学模型在确定反应温度和压力范围内反应的吉布斯自由能的最小化方面是可靠的。该模型遵循Marrero-Gani,Joback-Reid和Chen-Dinivahi-Jeng的方法估算纯组分的热力学性质。考虑到部分逸度系数来计算逸度,并使用Peng-Robinson状态方程和范德华混合规则。使用MATLAB通过最小二乘法分析来估算平衡时的摩尔分数,为了考虑反应热力学性质的非理想性,并找到使总吉布斯自由能最小化的平衡组成。同样,平衡反应的反应坐标与组成的已知值一起被估算,以便知道压力和温度对上述反应的影响。
更新日期:2021-03-16
中文翻译:

游离脂肪酸加氢脱氧以获得绿色柴油的最佳条件确定
在这项工作中,研究了绿色柴油的生产,该绿色柴油涉及植物油(例如油酸)的加氢脱氧反应,产生副产物CO或CO 2。该反应在氢饱和下进行以产生硬脂酸;随后,通过脱羰和脱羧途径生成庚烷,而随后通过十八烷的加氢脱氧获得十八烷。在十八烷的形成过程中,会获得水作为副产物,而十七烷的产生会导致CO和CO 2的产生。作为脱羰和脱羧反应的副产物。当考虑甘油三酸酯和游离酸的加氢处理中的产物和反应路线时,发现数学模型在确定反应温度和压力范围内反应的吉布斯自由能的最小化方面是可靠的。该模型遵循Marrero-Gani,Joback-Reid和Chen-Dinivahi-Jeng的方法估算纯组分的热力学性质。考虑到部分逸度系数来计算逸度,并使用Peng-Robinson状态方程和范德华混合规则。使用MATLAB通过最小二乘法分析来估算平衡时的摩尔分数,为了考虑反应热力学性质的非理想性,并找到使总吉布斯自由能最小化的平衡组成。同样,平衡反应的反应坐标与组成的已知值一起被估算,以便知道压力和温度对上述反应的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号