Nano Energy ( IF 16.8 ) Pub Date : 2021-01-16 , DOI: 10.1016/j.nanoen.2021.105767 HuangJingWei Li , Kang Liu , Junwei Fu , Kejun Chen , Kexin Yang , Yiyang Lin , Baopeng Yang , Qiyou Wang , Hao Pan , Zhoujun Cai , Hongmei Li , Maoqi Cao , Junhua Hu , Ying-Rui Lu , Ting-Shan Chan , Emiliano Cortés , Andrea Fratalocchi , Min Liu
|
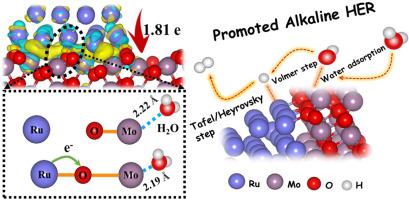
Electrocatalytic hydrogen evolution reaction (HER) in alkaline media is a promising electrochemical energy conversion strategy. Ruthenium (Ru) is an efficient catalyst with a desirable cost for HER, however, the sluggish H2O dissociation process, due to the low H2O adsorption on its surface, currently hampers the performances of this catalyst in alkaline HER. Herein, we demonstrate that the H2O adsorption improves significantly by the construction of Ru–O–Mo sites. We prepared Ru/MoO2 catalysts with Ru–O–Mo sites through a facile thermal treatment process and assessed the creation of Ru–O–Mo interfaces by transmission electron microscope (TEM) and extended X-ray absorption fine structure (EXAFS). By using Fourier-transform infrared spectroscopy (FTIR) and H2O adsorption tests, we proved Ru–O–Mo sites have tenfold stronger H2O adsorption ability than that of Ru catalyst. The catalysts with Ru–O–Mo sites exhibited a state-of-the-art overpotential of 16 mV at 10 mA cm–2 in 1 M KOH electrolyte, demonstrating a threefold reduction than the previous bests of Ru (59 mV) and commercial Pt (31 mV) catalysts. We proved the stability of these performances over 40 hours without decline. These results could open a new path for designing efficient and stable catalysts.
中文翻译:

配对的Ru-O-Mo团用于有效和稳定的碱性氢释放反应
碱性介质中的电催化放氢反应(HER)是一种有前途的电化学能量转化策略。钌(Ru)是一种有效的催化剂,对HER而言具有理想的成本,但是,由于其表面H 2 O的吸附量低,H 2 O的解离过程缓慢,目前阻碍了该催化剂在碱性HER中的性能。在本文中,我们证明通过Ru–O–Mo位点的构建,H 2 O吸附显着改善。我们准备了Ru / MoO 2具有Ru-O-Mo位置的催化剂通过便捷的热处理过程,并通过透射电子显微镜(TEM)和扩展的X射线吸收精细结构(EXAFS)评估了Ru-O-Mo界面的形成。通过傅里叶变换红外光谱(FTIR)和H 2 O吸附测试,我们证明Ru–O–Mo位置的H 2 O吸附能力比Ru催化剂强十倍。具有Ru–O–Mo位置的催化剂在10 mA cm –2处表现出16 mV的最新超电势在1 M KOH电解液中,比Ru(59 mV)和商用Pt(31 mV)催化剂的先前最佳性能降低了三倍。我们证明了这些性能在40个小时内的稳定性,而且没有下降。这些结果可以为设计高效,稳定的催化剂开辟新的道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号