Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2021-01-17 , DOI: 10.1016/j.cej.2021.128529 Nguyen Thi Thao Nguyen , Anh Quoc Khuong Nguyen , Min Sik Kim , Changha Lee , Jungwon Kim
|
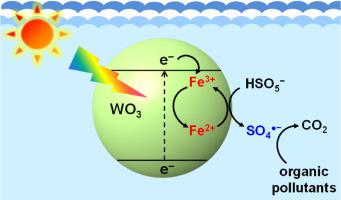
The effect of ferric ions (Fe3+) on the degradation of organic pollutants through the photocatalytic activation of peroxymonosulfate (PMS) was investigated. In the presence of Fe3+, the degradation of 4-chlorophenol (4-CP) was significantly enhanced in the PMS/tungsten oxide (WO3) system under visible light irradiation. The enhanced degradation efficiency by Fe3+ is primarily ascribed to the role of Fe3+ as an electron-transfer mediator, as it induces a cascadal electron transfer from WO3 to Fe3+ to PMS. The production of the sulfate radical (SO4 −) and its significant contribution to the degradation of 4-CP in the Fe3+/PMS/WO3 system were verified via electron paramagnetic resonance (EPR) spectroscopy and degradation experiments using radical scavengers (methanol and tert-butyl alcohol), respectively. The addition of Fe3+ to the PMS/WO3 system also significantly increased the degradation rate of other organic pollutants, such as phenol, 2,4-dimethylphenol, bisphenol A, sulfamethoxazole, sulfanilamide, and propranolol. The degradation efficiency of the Fe3+/PMS/WO3 system was largely maintained in multiple degradation cycles by the regular addition of PMS only. In addition, the Fe3+/PMS/WO3 system exhibited a higher degradation efficiency than the conventional cobaltous ion (Co2+)/PMS system at equal transition metal ion concentrations. The addition of Fe3+ for the purpose of enhancing the degradation efficiency is not restricted to the PMS activation system. This method can be applied to the activation of other oxyanions, such as peroxydisulfate (S2O82−) and bromate (BrO3−), for wastewater treatment.
−) and its significant contribution to the degradation of 4-CP in the Fe3+/PMS/WO3 system were verified via electron paramagnetic resonance (EPR) spectroscopy and degradation experiments using radical scavengers (methanol and tert-butyl alcohol), respectively. The addition of Fe3+ to the PMS/WO3 system also significantly increased the degradation rate of other organic pollutants, such as phenol, 2,4-dimethylphenol, bisphenol A, sulfamethoxazole, sulfanilamide, and propranolol. The degradation efficiency of the Fe3+/PMS/WO3 system was largely maintained in multiple degradation cycles by the regular addition of PMS only. In addition, the Fe3+/PMS/WO3 system exhibited a higher degradation efficiency than the conventional cobaltous ion (Co2+)/PMS system at equal transition metal ion concentrations. The addition of Fe3+ for the purpose of enhancing the degradation efficiency is not restricted to the PMS activation system. This method can be applied to the activation of other oxyanions, such as peroxydisulfate (S2O82−) and bromate (BrO3−), for wastewater treatment.
中文翻译:

Fe 3+作为电子转移介体对WO 3可见光下过氧化一硫酸盐活化的影响
研究了铁离子(Fe 3+)通过过氧单硫酸盐(PMS)的光催化活化对有机污染物降解的影响。在Fe 3+存在下,在可见光照射下,PMS /氧化钨(WO 3)体系中4-氯苯酚(4-CP)的降解显着增强。Fe 3+增强的降解效率主要归因于Fe 3+作为电子传递介体的作用,因为它诱导了从WO 3到Fe 3+到PMS的级联电子转移。生产硫酸根(SO 4  - )及其到4-CP中的Fe的降解显著贡献分别通过电子顺磁共振(EPR)光谱和使用自由基清除剂(甲醇和叔丁醇)的降解实验验证了3+ / PMS / WO 3系统。在PMS / WO 3系统中添加Fe 3+还可显着提高其他有机污染物的降解速度,例如苯酚,2,4-二甲基苯酚,双酚A,磺胺甲恶唑,磺胺和普萘洛尔。仅通过定期添加PMS,可以在多个降解循环中很大程度上保持Fe 3+ / PMS / WO 3系统的降解效率。另外,Fe 3+ / PMS / WO 3在相同的过渡金属离子浓度下,该系统显示出比常规钴离子(Co 2 +)/ PMS系统更高的降解效率。为了提高降解效率而添加Fe 3+并不限于PMS活化系统。这种方法可以被应用到其它含氧阴离子,如过二的活化(S 2 ö 8 2-)和溴酸盐(BRO 3 - ),用于废水处理。
- )及其到4-CP中的Fe的降解显著贡献分别通过电子顺磁共振(EPR)光谱和使用自由基清除剂(甲醇和叔丁醇)的降解实验验证了3+ / PMS / WO 3系统。在PMS / WO 3系统中添加Fe 3+还可显着提高其他有机污染物的降解速度,例如苯酚,2,4-二甲基苯酚,双酚A,磺胺甲恶唑,磺胺和普萘洛尔。仅通过定期添加PMS,可以在多个降解循环中很大程度上保持Fe 3+ / PMS / WO 3系统的降解效率。另外,Fe 3+ / PMS / WO 3在相同的过渡金属离子浓度下,该系统显示出比常规钴离子(Co 2 +)/ PMS系统更高的降解效率。为了提高降解效率而添加Fe 3+并不限于PMS活化系统。这种方法可以被应用到其它含氧阴离子,如过二的活化(S 2 ö 8 2-)和溴酸盐(BRO 3 - ),用于废水处理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号