Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2021-01-16 , DOI: 10.1016/j.bmc.2021.116017 Nibedita Ghosh 1 , Lal Mohan Kundu 2
|
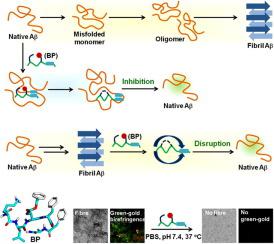
Accumulation and deposition of misfolded amyloid β (Aβ) peptide outside the nerve cells are one of the major causes of Alzheimer’s disease (AD). To date, one of the promising therapeutic strategies for AD is to block the early steps associated with the aggregation of Aβ peptide. We have developed synthetic breaker peptides derived from the original Aβ sequences that undergo self-cyclization in situ. We have focussed and replaced Val-18 (of Aβ) by side-chain modified glutamic acid (Glu-OBn) to generate adequate turn through in-situ peptide cyclization to disrupt the β-sheet structure of Aβ. The disruption of amyloid fibril formation and the mechanism of the ‘inhibition of aggregation’ were studied by various biophysical methods, such as ThT-assay, TEM, Congo-red birefringence study. CD and FTIR spectroscopy were used to characterize the conformational change during the aggregation process. Results suggest that designed breaker peptides may be useful to inhibit and disrupt not only Aβ peptide but related peptides that undergo aggregation.
中文翻译:

原位侧链肽环化作为对抗淀粉样蛋白聚集肽的破坏策略
错误折叠的淀粉样蛋白 β (Aβ) 肽在神经细胞外的积累和沉积是阿尔茨海默病 (AD) 的主要原因之一。迄今为止,AD 有希望的治疗策略之一是阻断与 Aβ 肽聚集相关的早期步骤。我们开发了源自原始 Aβ 序列的合成破坏肽,这些序列在原位进行自环化。我们已经集中并用侧链修饰的谷氨酸 (Glu-OBn) 替代了 Val-18(Aβ),以通过原位产生足够的转向肽环化破坏 Aβ 的 β-折叠结构。通过各种生物物理方法,如 ThT 测定、TEM、刚果红双折射研究,研究了淀粉样原纤维形成的破坏和“抑制聚集”的机制。CD和FTIR光谱用于表征聚集过程中的构象变化。结果表明,设计的破坏肽不仅可用于抑制和破坏 Aβ 肽,还可用于抑制和破坏发生聚集的相关肽。



























 京公网安备 11010802027423号
京公网安备 11010802027423号