Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2021-01-16 , DOI: 10.1016/j.bmc.2021.116019 Ikumi Kuriwaki 1 , Minoru Kameda 1 , Kazuhiko Iikubo 1 , Hiroyuki Hisamichi 1 , Yuichiro Kawamoto 1 , Shigetoshi Kikuchi 1 , Hiroyuki Moritomo 1 , Yutaka Kondoh 1 , Tadashi Terasaka 1 , Yasushi Amano 1 , Yukihiro Tateishi 1 , Yuka Echizen 1 , Yoshinori Iwai 2 , Atsushi Noda 2 , Hiroshi Tomiyama 2 , Taisuke Nakazawa 1 , Masaaki Hirano 1
|
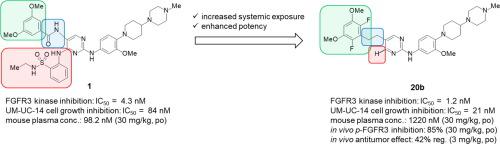
Fibroblast growth factor receptor 3 (FGFR3) is an attractive therapeutic target for the treatment of patients with bladder cancer harboring genetic alterations in FGFR3. We identified pyrimidine derivative 20b, which induced tumor regression following oral administration to a bladder cancer xenograft mouse model. Compound 20b was discovered by optimizing lead compound 1, which we reported previously. Specifically, reducing the molecular size of the substituent at the 4-position and replacing the linker of the 5-position in the pyrimidine scaffold resulted in an increase in systemic exposure. Furthermore, introduction of two fluorine atoms into the 3,5-dimethoxyphenyl ring enhanced FGFR3 inhibitory activity. Molecular dynamics (MD) simulation of 20b suggested that the fluorine atom interacts with the main chain NH moiety of Asp635 via a hydrogen bond.
中文翻译:

嘧啶衍生物作为有效和口服活性 FGFR3 抑制剂的合成和构效关系,具有增加的全身暴露和增强的体外效力
成纤维细胞生长因子受体 3 (FGFR3) 是治疗具有 FGFR3 基因改变的膀胱癌患者的有吸引力的治疗靶点。我们鉴定了嘧啶衍生物20b,其在向膀胱癌异种移植小鼠模型口服给药后诱导肿瘤消退。化合物20b是通过优化我们之前报道的先导化合物1发现的。具体而言,减少 4 位取代基的分子大小并替换嘧啶支架中 5 位的接头导致全身暴露增加。此外,将两个氟原子引入 3,5-二甲氧基苯环增强了 FGFR3 抑制活性。分子动力学 (MD) 模拟图 20b表明氟原子通过氢键与 Asp635 的主链 NH 部分相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号