当前位置:
X-MOL 学术
›
RSC Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bypassing the requirement for aminoacyl-tRNA by a cyclodipeptide synthase enzyme
RSC Chemical Biology ( IF 4.2 ) Pub Date : 2021-1-15 , DOI: 10.1039/d0cb00142b Christopher J Harding 1 , Emmajay Sutherland 1 , Jane G Hanna 2 , Douglas R Houston 3 , Clarissa M Czekster 1
RSC Chemical Biology ( IF 4.2 ) Pub Date : 2021-1-15 , DOI: 10.1039/d0cb00142b Christopher J Harding 1 , Emmajay Sutherland 1 , Jane G Hanna 2 , Douglas R Houston 3 , Clarissa M Czekster 1
Affiliation
|
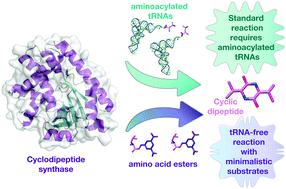
Cyclodipeptide synthases (CDPSs) produce a variety of cyclic dipeptide products by utilising two aminoacylated tRNA substrates. We sought to investigate the minimal requirements for substrate usage in this class of enzymes as the relationship between CDPSs and their substrates remains elusive. Here, we investigated the Bacillus thermoamylovorans enzyme, BtCDPS, which synthesises cyclo(L-Leu–L-Leu). We systematically tested where specificity arises and, in the process, uncovered small molecules (activated amino esters) that will suffice as substrates, although catalytically poor. We solved the structure of BtCDPS to 1.7 Å and combining crystallography, enzymatic assays and substrate docking experiments propose a model for how the minimal substrates interact with the enzyme. This work is the first report of a CDPS enzyme utilizing a molecule other than aa-tRNA as a substrate; providing insights into substrate requirements and setting the stage for the design of improved simpler substrates.
中文翻译:

通过环二肽合酶绕过对氨酰基-tRNA 的需求
环二肽合酶 (CDPS) 利用两种氨酰化 tRNA 底物产生多种环状二肽产物。我们试图研究此类酶对底物使用的最低要求,因为 CDPS 与其底物之间的关系仍然难以捉摸。在这里,我们研究了热解淀粉芽孢杆菌酶 BtCDPS,它合成环( L -Leu- L -Leu)。我们系统地测试了特异性出现的位置,并在此过程中发现了足以作为底物的小分子(活化的氨基酯),尽管催化能力较差。我们将 BtCDPS 的结构解析为 1.7 Å,并结合晶体学、酶分析和底物对接实验提出了最小底物如何与酶相互作用的模型。这项工作是首次报道 CDPS 酶利用 aa-tRNA 以外的分子作为底物;提供对基材要求的深入了解,并为改进的更简单基材的设计奠定基础。
更新日期:2021-01-15
中文翻译:

通过环二肽合酶绕过对氨酰基-tRNA 的需求
环二肽合酶 (CDPS) 利用两种氨酰化 tRNA 底物产生多种环状二肽产物。我们试图研究此类酶对底物使用的最低要求,因为 CDPS 与其底物之间的关系仍然难以捉摸。在这里,我们研究了热解淀粉芽孢杆菌酶 BtCDPS,它合成环( L -Leu- L -Leu)。我们系统地测试了特异性出现的位置,并在此过程中发现了足以作为底物的小分子(活化的氨基酯),尽管催化能力较差。我们将 BtCDPS 的结构解析为 1.7 Å,并结合晶体学、酶分析和底物对接实验提出了最小底物如何与酶相互作用的模型。这项工作是首次报道 CDPS 酶利用 aa-tRNA 以外的分子作为底物;提供对基材要求的深入了解,并为改进的更简单基材的设计奠定基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号