当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The use of MM/QM calculations of 13C chemical shifts in the conformational analysis of some monosaccharides and sucrose
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2020-12-7 , DOI: 10.1039/d0nj04227g Raymond J. Abraham 1, 2, 3, 4 , M. Ashley Cooper 1, 2, 3, 4 , Matthew Reid 4, 5, 6
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2020-12-7 , DOI: 10.1039/d0nj04227g Raymond J. Abraham 1, 2, 3, 4 , M. Ashley Cooper 1, 2, 3, 4 , Matthew Reid 4, 5, 6
Affiliation
|
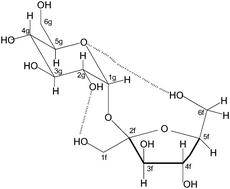
The 13C NMR chemical shifts of some common pyranose monosaccharides in D2O solution were predicted using a combined molecular mechanics (Pcmod 9.1/MMFF94) and ab initio (GIAO (B3LYP/DFT, 6-31G(d))) model. This method has been successfully used for a range of organic molecules in CDCl3 solution but has not previously been used for aqueous solutions. The method gave such good agreement with experiment that the populations of the conformers at C6 of the monosaccharides could be obtained. This has been attempted previously by various methods with diverse results. In all the compounds studied the α and β anomers have similar populations. In glucose the populations of the GG and GT conformers are ca equal with TG a minor component, in galactose GT has the largest population and GG the smallest, in mannose GG is preferred with TG a minor component and in talose GT and TG are favoured with GG a minor constituent. The results are in general agreement with previous work. To our knowledge this is the first time that 13C shifts have been used to determine sugar conformations in solution. The conformation of sucrose in D2O solution was examined by both 1H and 13C NMR. The complete analysis of the 1H NMR spectrum has been achieved and provides information on the preferred conformations of the CH2OH fragments at G6 (GG) and F6 (GG, GT). Calculations of the 13C shifts confirm these results and show also that the conformation at F1 is mainly GG plus TG. The sucrose conformational profile in solution includes the crystal structure together with the above conformations at F1 and F6.
中文翻译:

MM / QM计算13C化学位移在某些单糖和蔗糖构象分析中的应用
使用组合分子力学(Pcmod 9.1 / MMFF94)和从头算(GIAO(B3LYP / DFT,6-31G(d)))模型预测了D 2 O溶液中一些常见的吡喃糖单糖的13 C NMR化学位移。该方法已成功用于CDCl 3溶液中的多种有机分子,但先前尚未用于水溶液。该方法与实验吻合得很好,以至于C 6的构象体总数可获得单糖中的一个。先前已经通过各种方法尝试了这种方法,但结果各不相同。在所有研究的化合物中,α和β端基异构体的种群相似。在葡萄糖中,GG和GT构象异构体的群体与TG是次要组分,在半乳糖GT中具有最大的群体,而GG在最小群体中,甘露糖GG优选与TG作为次要组分,而在talose中GT和TG则优选GG是次要组成部分。结果与先前的工作基本一致。据我们所知,这是第一次使用13 C变换确定溶液中的糖构象。1 H和13均检查了D 2 O溶液中蔗糖的构象1 H NMR。已经完成了1 H NMR光谱的完整分析,并提供了有关G 6(GG)和F6(GG,GT)上CH 2 OH片段优选构象的信息。13 C位移的计算证实了这些结果,并且还表明F1处的构象主要是GG加TG。溶液中的蔗糖构象轮廓包括晶体结构以及在F1和F6的上述构象。
更新日期:2021-01-15
中文翻译:

MM / QM计算13C化学位移在某些单糖和蔗糖构象分析中的应用
使用组合分子力学(Pcmod 9.1 / MMFF94)和从头算(GIAO(B3LYP / DFT,6-31G(d)))模型预测了D 2 O溶液中一些常见的吡喃糖单糖的13 C NMR化学位移。该方法已成功用于CDCl 3溶液中的多种有机分子,但先前尚未用于水溶液。该方法与实验吻合得很好,以至于C 6的构象体总数可获得单糖中的一个。先前已经通过各种方法尝试了这种方法,但结果各不相同。在所有研究的化合物中,α和β端基异构体的种群相似。在葡萄糖中,GG和GT构象异构体的群体与TG是次要组分,在半乳糖GT中具有最大的群体,而GG在最小群体中,甘露糖GG优选与TG作为次要组分,而在talose中GT和TG则优选GG是次要组成部分。结果与先前的工作基本一致。据我们所知,这是第一次使用13 C变换确定溶液中的糖构象。1 H和13均检查了D 2 O溶液中蔗糖的构象1 H NMR。已经完成了1 H NMR光谱的完整分析,并提供了有关G 6(GG)和F6(GG,GT)上CH 2 OH片段优选构象的信息。13 C位移的计算证实了这些结果,并且还表明F1处的构象主要是GG加TG。溶液中的蔗糖构象轮廓包括晶体结构以及在F1和F6的上述构象。











































 京公网安备 11010802027423号
京公网安备 11010802027423号