当前位置:
X-MOL 学术
›
J. Mater. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeted delivery and controlled release of doxorubicin to cancer cells by smart ATP-responsive Y-shaped DNA structure-capped mesoporous silica nanoparticles
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2021-1-15 , DOI: 10.1039/d0tb01960g Elnaz Bagheri 1, 2, 3, 4, 5 , Mona Alibolandi 1, 2, 3, 4, 5 , Khalil Abnous 3, 4, 5, 6, 7 , Seyed Mohammad Taghdisi 2, 3, 4, 5, 8 , Mohammad Ramezani 1, 2, 3, 4, 5
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2021-1-15 , DOI: 10.1039/d0tb01960g Elnaz Bagheri 1, 2, 3, 4, 5 , Mona Alibolandi 1, 2, 3, 4, 5 , Khalil Abnous 3, 4, 5, 6, 7 , Seyed Mohammad Taghdisi 2, 3, 4, 5, 8 , Mohammad Ramezani 1, 2, 3, 4, 5
Affiliation
|
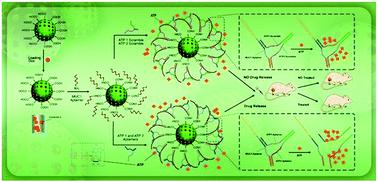
In this study, a dual-receptor doxorubicin-targeted delivery system based on mesoporous silica nanoparticles (MSNs) modified with mucine-1 and ATP aptamers (DOX@MSNs-Apts) was developed. An amine-modified mucine-1 (MUC1) aptamer was covalently anchored on the surface of carboxyl-functionalized MSNs. Then, ATP aptamers (ATP1 and ATP2 aptamers) were immobilized on the surface of MSNs through partial hybridization with the MUC1 aptamer by forming a Y-shaped DNA structure on the MSNs surface (DOX@MSNs-Apts) as a gatekeeper. The developed DOX@MSNs-Apts exhibited high DOX loading capacity. In addition, it indicated an ATP-responsive feature, leading to the release of DOX in the environment with high ATP concentration (10 mM), similar to the intracellular environment of tumor cells. This property demonstrated that anticancer drug (DOX) could be entrapped inside the nanocarrier with nearly no leakage in blood and a very low concentration of ATP (1 μM). It was found that after the internalization of DOX@MSNs-MUC1 by cancer cells via the MUC1 receptor-mediated endocytosis, the ATP aptamers left the surface of the nanocarrier, allowing for rapid DOX release. DOX@MSNs-Apts indicated higher cellular uptake in MCF-7 and C26 cancer cells (MUC1+), rather than CHO cells (MUC1−). The in vitro cytotoxicity and the in vivo antitumor efficacy of DOX@MSNs-Apts showed greater cytotoxicity than the nanoparticles decorated with scrambled ATP aptamers (DOX@MSNs-Apts scrambled) in C26 and MCF-7 cell lines (MUC1+). The biodistribution and in vivo anticancer efficacy on the C26 tumor bearing mice indicated that the DOX@MSNs-Apts had a higher tumor accumulation and superior tumor growth inhibitory effect compared to free DOX and their scrambled aptamers, DOX@MSNs-Apts scrambled. Overall, the obtained results indicated that the prepared smart platform could reveal new insights into the treatment of cancer.
中文翻译:

通过智能的ATP响应Y型DNA结构封端的介孔二氧化硅纳米粒子靶向阿霉素向癌细胞的递送和控制释放
在这项研究中,开发了一种以mucine-1和ATP适体(DOX @ MSNs-Apts)修饰的介孔二氧化硅纳米粒子(MSNs)为基础的双受体阿霉素靶向递送系统。胺修饰的mucine-1(MUC1)适体共价锚定在羧基官能化MSN的表面上。然后,通过与MUC1适体部分杂交,通过在MSNs表面形成一个Y形DNA结构(DOX @ MSNs-Apts)作为网守,将ATP适体(ATP1和ATP2适体)固定在MSNs表面。发达的DOX @ MSNs-Apts具有很高的DOX加载能力。此外,它表明了ATP响应特性,导致在高ATP浓度(10 mM)的环境中释放DOX,类似于肿瘤细胞的细胞内环境。该性质表明,抗癌药(DOX)可以被包裹在纳米载体内部,几乎没有血液泄漏,并且ATP的浓度非常低(1μM)。发现在DOX @ MSNs-MUC1被癌细胞内化后通过MUC1受体介导的内吞作用,ATP适体离开了纳米载体的表面,可以快速释放DOX。DOX @的MSN-普茨在MCF-7和C26癌细胞(MUC1指示更高的细胞摄取+),而不是CHO细胞(MUC1 - )。在体外细胞毒性和体内DOX的抗肿瘤功效@的MSN-普茨显示出比装饰有在C26和MCF-7细胞系(MUC1加扰ATP适体(DOX @的MSN-普茨加扰)纳米颗粒更大的细胞毒性+)。生物分布和体内对C26荷瘤小鼠的抗癌功效表明,与游离DOX及其加扰的适体DOX @ MSNs-Apts相比,DOX @ MSNs-Apts具有更高的肿瘤蓄积和优异的肿瘤生长抑制作用。总体而言,获得的结果表明,准备好的智能平台可以揭示癌症治疗的新见解。
更新日期:2021-01-15
中文翻译:

通过智能的ATP响应Y型DNA结构封端的介孔二氧化硅纳米粒子靶向阿霉素向癌细胞的递送和控制释放
在这项研究中,开发了一种以mucine-1和ATP适体(DOX @ MSNs-Apts)修饰的介孔二氧化硅纳米粒子(MSNs)为基础的双受体阿霉素靶向递送系统。胺修饰的mucine-1(MUC1)适体共价锚定在羧基官能化MSN的表面上。然后,通过与MUC1适体部分杂交,通过在MSNs表面形成一个Y形DNA结构(DOX @ MSNs-Apts)作为网守,将ATP适体(ATP1和ATP2适体)固定在MSNs表面。发达的DOX @ MSNs-Apts具有很高的DOX加载能力。此外,它表明了ATP响应特性,导致在高ATP浓度(10 mM)的环境中释放DOX,类似于肿瘤细胞的细胞内环境。该性质表明,抗癌药(DOX)可以被包裹在纳米载体内部,几乎没有血液泄漏,并且ATP的浓度非常低(1μM)。发现在DOX @ MSNs-MUC1被癌细胞内化后通过MUC1受体介导的内吞作用,ATP适体离开了纳米载体的表面,可以快速释放DOX。DOX @的MSN-普茨在MCF-7和C26癌细胞(MUC1指示更高的细胞摄取+),而不是CHO细胞(MUC1 - )。在体外细胞毒性和体内DOX的抗肿瘤功效@的MSN-普茨显示出比装饰有在C26和MCF-7细胞系(MUC1加扰ATP适体(DOX @的MSN-普茨加扰)纳米颗粒更大的细胞毒性+)。生物分布和体内对C26荷瘤小鼠的抗癌功效表明,与游离DOX及其加扰的适体DOX @ MSNs-Apts相比,DOX @ MSNs-Apts具有更高的肿瘤蓄积和优异的肿瘤生长抑制作用。总体而言,获得的结果表明,准备好的智能平台可以揭示癌症治疗的新见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号