当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reversible PtII–CH3 deuteration without methane loss: metal–ligand cooperation vs. ligand-assisted PtII-protonation
Chemical Science ( IF 7.6 ) Pub Date : 2021-1-5 , DOI: 10.1039/d0sc06518h Shrinwantu Pal 1 , Kyoko Nozaki 1 , Andrei N Vedernikov 2 , Jennifer A Love 3
Chemical Science ( IF 7.6 ) Pub Date : 2021-1-5 , DOI: 10.1039/d0sc06518h Shrinwantu Pal 1 , Kyoko Nozaki 1 , Andrei N Vedernikov 2 , Jennifer A Love 3
Affiliation
|
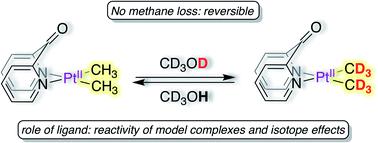
Di(2-pyridyl)ketone dimethylplatinum(II), (dpk)PtII(CH3)2, reacts with CD3OD at 25 °C to undergo complete deuteration of Pt–CH3 fragments in ∼5 h without loss of methane to form (dpk)PtII(CD3)2 in virtually quantitative yield. The deuteration can be reversed by dissolution in CH3OH or CD3OH. Kinetic analysis and isotope effects, together with support from density functional theory calculations indicate a metal–ligand cooperative mechanism wherein DPK enables Pt–CH3 deuteration by allowing non-rate-limiting protonation of PtII by CD3OD. In contrast, other model di(2-pyridyl) ligands enable rate-limiting protonation of PtII, resulting in non-rate-limiting C–H(D) reductive coupling. Owing to its electron-poor nature, following complete deuteration, DPK can be dissociated from the PtII-centre, furnishing [(CD3)2PtII(μ-SMe2)]2 as the perdeutero analogue of [(CH3)2PtII(μ-SMe2)]2, a commonly used PtII-precursor.
中文翻译:

可逆的PtII-CH3氘化而没有甲烷损失:金属-配体配合与配体辅助的PtII-质子化
二(2-吡啶基)酮二甲基铂(II),(dpk)Pt II(CH 3)2在25°C下与CD 3 OD反应,在约5小时内对Pt–CH 3片段进行完全氘化,而不会损失甲烷以几乎定量的产率形成(dpk)Pt II(CD 3)2。氘化可以通过溶解在CH 3 OH或CD 3 OH中来逆转。动力学分析和同位素效应以及密度泛函理论计算的支持表明了一种金属-配体协同机制,其中DPK通过允许Pt的非速率限制质子化作用使Pt-CH 3氘化II通过CD 3 OD。相比之下,其他模型二(2-吡啶基)配体可实现Pt II的限速质子化,从而导致非限速的CH(D)还原偶联。由于它的贫电子的性质,以下完整氘,DPK可以从铂上解离II -Centre,家具[(CD 3) 2的Pt II(μ-SMe的2)] 2为[(CH的全氘类似物3) 2的Pt II(μ-SMe的2)] 2,通常使用的铂II -precursor。
更新日期:2021-01-15
中文翻译:

可逆的PtII-CH3氘化而没有甲烷损失:金属-配体配合与配体辅助的PtII-质子化
二(2-吡啶基)酮二甲基铂(II),(dpk)Pt II(CH 3)2在25°C下与CD 3 OD反应,在约5小时内对Pt–CH 3片段进行完全氘化,而不会损失甲烷以几乎定量的产率形成(dpk)Pt II(CD 3)2。氘化可以通过溶解在CH 3 OH或CD 3 OH中来逆转。动力学分析和同位素效应以及密度泛函理论计算的支持表明了一种金属-配体协同机制,其中DPK通过允许Pt的非速率限制质子化作用使Pt-CH 3氘化II通过CD 3 OD。相比之下,其他模型二(2-吡啶基)配体可实现Pt II的限速质子化,从而导致非限速的CH(D)还原偶联。由于它的贫电子的性质,以下完整氘,DPK可以从铂上解离II -Centre,家具[(CD 3) 2的Pt II(μ-SMe的2)] 2为[(CH的全氘类似物3) 2的Pt II(μ-SMe的2)] 2,通常使用的铂II -precursor。











































 京公网安备 11010802027423号
京公网安备 11010802027423号