当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereoselective Synthesis of Terminal Bromo-Substituted Propargylamines via Generation of Lithium Bromoacetylide and Addition to Chiral N-tert-Butanesulfinyl Aldimines
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-01-15 , DOI: 10.1021/acs.joc.0c02697 Charles S Jolly 1 , Emma Kochanowski 1 , Cayden J Dodd 1 , Savannah J Post 1 , Harrison M Hill 1 , Mark Turlington 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-01-15 , DOI: 10.1021/acs.joc.0c02697 Charles S Jolly 1 , Emma Kochanowski 1 , Cayden J Dodd 1 , Savannah J Post 1 , Harrison M Hill 1 , Mark Turlington 1
Affiliation
|
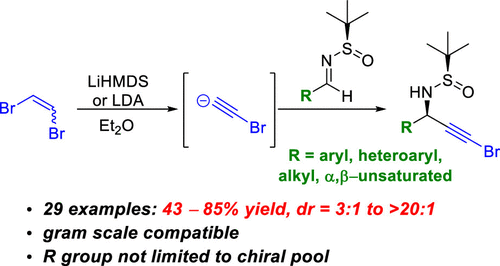
The stereoselective synthesis of terminal bromo-substituted propargylamines via in situ generation of lithium bromoacetylide from 1,2-dibromoethene and addition to Ellman chiral N-tert-butanesulfinyl aldimines is reported. Modest to good yields (43–85%) and diastereoselectivity (dr = 3:1 to >20:1) were achieved for a range of aryl, heteroaryl, alkyl, and α,β-unsaturated substrates. Terminal bromo-substituted propargylamines prepared via this method can be directly used in the frequently employed Cadiot–Chodkiewicz coupling to produce functionalized diynes. The method reported here increases the structural diversity of chiral terminal bromo-substituted propargylamines that can be readily synthesized as previous methods for the stereoselective synthesis of these compounds rely on amino acid precursors from the chiral pool.
中文翻译:

通过锂Bromoacetylide和加成生成终端溴-取代的炔丙胺的非对映选择性合成在手性ñ -叔-Butanesulfinyl醛亚胺
通过从1,2-二溴乙烯原位生成溴乙炔锂并加到Ellman手性N - tert上立体合成末端溴取代的炔丙基胺。报道了-丁亚磺酰基醛胺。对于一系列芳基,杂芳基,烷基和α,β-不饱和底物,可实现中等至良好的收率(43-85%)和非对映选择性(dr = 3:1至> 20:1)。通过这种方法制备的末端溴取代的炔丙基胺可直接用于经常使用的Cadiot–Chodkiewicz偶联反应中,以生成功能化的二炔。本文报道的方法增加了手性末端溴取代的炔丙基胺的结构多样性,可以容易地合成这些化合物,因为这些化合物的立体选择性合成的先前方法依赖于手性库中的氨基酸前体。
更新日期:2021-02-05
中文翻译:

通过锂Bromoacetylide和加成生成终端溴-取代的炔丙胺的非对映选择性合成在手性ñ -叔-Butanesulfinyl醛亚胺
通过从1,2-二溴乙烯原位生成溴乙炔锂并加到Ellman手性N - tert上立体合成末端溴取代的炔丙基胺。报道了-丁亚磺酰基醛胺。对于一系列芳基,杂芳基,烷基和α,β-不饱和底物,可实现中等至良好的收率(43-85%)和非对映选择性(dr = 3:1至> 20:1)。通过这种方法制备的末端溴取代的炔丙基胺可直接用于经常使用的Cadiot–Chodkiewicz偶联反应中,以生成功能化的二炔。本文报道的方法增加了手性末端溴取代的炔丙基胺的结构多样性,可以容易地合成这些化合物,因为这些化合物的立体选择性合成的先前方法依赖于手性库中的氨基酸前体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号