当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigation of Mixed MEA-Based Solvents Featuring Ionic Liquids and NMP for CO2 Capture: Experimental Measurement of CO2 Solubility and Thermophysical Properties
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-01-15 , DOI: 10.1021/acs.jced.0c00618 Mojgan Ebrahiminejadhasanabadi 1 , Wayne Michael Nelson 1 , Paramespri Naidoo 1 , Amir H. Mohammadi 1 , Deresh Ramjugernath 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-01-15 , DOI: 10.1021/acs.jced.0c00618 Mojgan Ebrahiminejadhasanabadi 1 , Wayne Michael Nelson 1 , Paramespri Naidoo 1 , Amir H. Mohammadi 1 , Deresh Ramjugernath 1
Affiliation
|
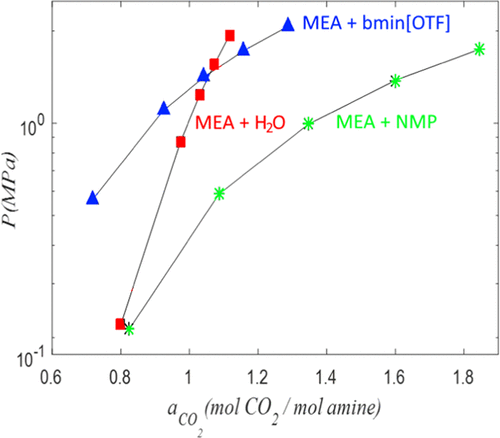
Alkanolamine solutions are used in the industry worldwide to remove carbon dioxide and hydrogen sulfide from natural gas, but such technologies have disadvantages. This study aimed to investigate the new hybrid amine solvents for carbon dioxide capture and their potential to reduce the disadvantages of amine technology. The solubility of carbon dioxide in hybrid solvents with different mixtures of monoethanolamine, water, n-methyl-2-pyrrolidone, 1-butyl-3-methylimidazolium trifluoromethanesulfonate, and 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide was measured at temperatures of 298.15 and 313.15 K by a static synthetic method. The viscosity, density, speed of sound, and evaporation rates for the hybrid solvents were also measured. Experimental observations indicate that the solubility of carbon dioxide in a hybrid solvent of n-methyl-2-pyrrolidone + monoethanolamine was greater than that of the aqueous amine solvent, except at low pressures depending on the concentration of amine. The addition of an ionic liquid to the amine solution reduced the volatility of the solvent and decelerated the evaporation rate of amine, while the loss of ionic liquid was almost zero. However, the addition of an ionic liquid to n-methyl-2-pyrrolidone-containing monoethanolamine solvent or the aqueous monoethanolamine solvent decreased the solubility of carbon dioxide and increased the viscosity of the solution.
中文翻译:

具有离子液体和NMP的混合MEA基溶剂对CO 2捕集的研究:CO 2溶解度和热物理性质的实验测量
链烷醇胺溶液在世界范围内的工业中用于从天然气中去除二氧化碳和硫化氢,但是这种技术具有缺点。这项研究旨在研究用于捕获二氧化碳的新型杂化胺溶剂及其在减少胺技术缺点方面的潜力。二氧化碳在单乙醇胺,水,n通过静态合成方法在298.15和313.15K的温度下测量-甲基-2-吡咯烷酮,1-丁基-3-甲基咪唑三氟甲磺酸盐和1-丁基-3-甲基咪唑双氟(三氟甲磺酰基)酰亚胺。还测量了混合溶剂的粘度,密度,声速和蒸发速率。实验观察表明,二氧化碳在正甲基-2-吡咯烷酮+单乙醇胺混合溶剂中的溶解度大于胺水溶液,但在低压下取决于胺的浓度除外。向胺溶液中添加离子液体可降低溶剂的挥发性,并降低胺的蒸发速率,而离子液体的损失几乎为零。但是,向其中添加了离子液体含正甲基-2-吡咯烷酮的单乙醇胺溶剂或含水单乙醇胺溶剂降低了二氧化碳的溶解度并增加了溶液的粘度。
更新日期:2021-02-11
中文翻译:

具有离子液体和NMP的混合MEA基溶剂对CO 2捕集的研究:CO 2溶解度和热物理性质的实验测量
链烷醇胺溶液在世界范围内的工业中用于从天然气中去除二氧化碳和硫化氢,但是这种技术具有缺点。这项研究旨在研究用于捕获二氧化碳的新型杂化胺溶剂及其在减少胺技术缺点方面的潜力。二氧化碳在单乙醇胺,水,n通过静态合成方法在298.15和313.15K的温度下测量-甲基-2-吡咯烷酮,1-丁基-3-甲基咪唑三氟甲磺酸盐和1-丁基-3-甲基咪唑双氟(三氟甲磺酰基)酰亚胺。还测量了混合溶剂的粘度,密度,声速和蒸发速率。实验观察表明,二氧化碳在正甲基-2-吡咯烷酮+单乙醇胺混合溶剂中的溶解度大于胺水溶液,但在低压下取决于胺的浓度除外。向胺溶液中添加离子液体可降低溶剂的挥发性,并降低胺的蒸发速率,而离子液体的损失几乎为零。但是,向其中添加了离子液体含正甲基-2-吡咯烷酮的单乙醇胺溶剂或含水单乙醇胺溶剂降低了二氧化碳的溶解度并增加了溶液的粘度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号