当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural Insights into a Novel Esterase from the East Pacific Rise and Its Improved Thermostability by a Semirational Design
Journal of Agricultural and Food Chemistry ( IF 6.1 ) Pub Date : 2021-01-14 , DOI: 10.1021/acs.jafc.0c06338 Chunhua Zhu 1, 2, 3 , Yayu Chen 4 , Michail N. Isupov 5 , Jennifer A. Littlechild 5 , Lifang Sun 4 , Xiaodong Liu 6, 7 , Qianchao Wang 1, 2 , Hui Gong 6 , Panpan Dong 4 , Na Zhang 4 , Yunkun Wu 4
Journal of Agricultural and Food Chemistry ( IF 6.1 ) Pub Date : 2021-01-14 , DOI: 10.1021/acs.jafc.0c06338 Chunhua Zhu 1, 2, 3 , Yayu Chen 4 , Michail N. Isupov 5 , Jennifer A. Littlechild 5 , Lifang Sun 4 , Xiaodong Liu 6, 7 , Qianchao Wang 1, 2 , Hui Gong 6 , Panpan Dong 4 , Na Zhang 4 , Yunkun Wu 4
Affiliation
|
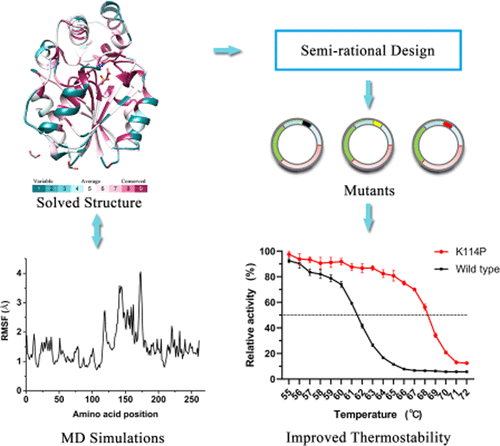
Lipolytic enzymes are essential biocatalysts in food processing as well as pharmaceutical and pesticide industries, catalyzing the cleavage of ester bonds in a variety of acyl chain substrates. Here, we report the crystal structure of an esterase from the deep-sea hydrothermal vent of the East Pacific Rise (EprEst). The X-ray structure of EprEst in complex with the ligand, acetate, has been determined at 2.03 Å resolution. The structure reveals a unique spatial arrangement and orientation of the helix cap domain and α/β hydrolase domain, which form a substrate pocket with preference for short-chain acyl groups. Molecular docking analysis further demonstrated that the active site pocket could accommodate p-nitrophenyl (pNP) carboxyl ligands of varying lengths (≤6 C atoms), with pNP-butyrate ester predicted to have the highest binding affinity. Additionally, the semirational design was conducted to improve the thermostability of EprEst by enzyme engineering based on the established structure and multiple sequence alignment. A mutation, K114P, introduced in the hinge region of the esterase, which displayed increased thermostability and enzyme activity. Collectively, the structural and functional data obtained herein could be used as basis for further protein engineering to ultimately expand the scope of industrial applications of marine-derived lipolytic enzymes.
中文翻译:

通过半定量设计对东太平洋崛起的新型酯酶及其热稳定性进行改进的结构见解
脂解酶是食品加工以及制药和农药行业中必不可少的生物催化剂,可催化各种酰基链底物中酯键的裂解。在这里,我们报告了东太平洋上升(EprEst)深海热液喷口的酯酶晶体结构。已测定EprEst与配体乙酸盐的络合物的X射线结构,分辨率为2.03Å。该结构揭示了螺旋帽结构域和α/β水解酶结构域的独特空间排列和方向,这些结构和结构形成了底物袋,偏爱短链酰基。分子对接分析进一步表明,活性位点口袋可以容纳长度(≤6 C原子)不同的对硝基苯基(p NP)羧基配体,p NP-丁酸酯预计具有最高的结合亲和力。另外,基于建立的结构和多序列比对,通过酶工程进行了半理性设计以提高EprEst的热稳定性。突变,K114P,引入酯酶的铰链区,显示出增加的热稳定性和酶活性。总体而言,本文获得的结构和功能数据可用作进一步蛋白质工程的基础,以最终扩大海洋衍生的脂解酶的工业应用范围。
更新日期:2021-01-27
中文翻译:

通过半定量设计对东太平洋崛起的新型酯酶及其热稳定性进行改进的结构见解
脂解酶是食品加工以及制药和农药行业中必不可少的生物催化剂,可催化各种酰基链底物中酯键的裂解。在这里,我们报告了东太平洋上升(EprEst)深海热液喷口的酯酶晶体结构。已测定EprEst与配体乙酸盐的络合物的X射线结构,分辨率为2.03Å。该结构揭示了螺旋帽结构域和α/β水解酶结构域的独特空间排列和方向,这些结构和结构形成了底物袋,偏爱短链酰基。分子对接分析进一步表明,活性位点口袋可以容纳长度(≤6 C原子)不同的对硝基苯基(p NP)羧基配体,p NP-丁酸酯预计具有最高的结合亲和力。另外,基于建立的结构和多序列比对,通过酶工程进行了半理性设计以提高EprEst的热稳定性。突变,K114P,引入酯酶的铰链区,显示出增加的热稳定性和酶活性。总体而言,本文获得的结构和功能数据可用作进一步蛋白质工程的基础,以最终扩大海洋衍生的脂解酶的工业应用范围。

































 京公网安备 11010802027423号
京公网安备 11010802027423号