当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a Non-IgG PD-1/PD-L1 Inhibitor by in Silico Mutagenesis and an In-Cell Protein–Protein Interaction Assay
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2021-01-15 , DOI: 10.1021/acschembio.0c00817 Boyang Ning 1, 2 , Xueli Ren 1 , Kyoji Hagiwara 1 , Shinji Takeoka 1, 2, 3 , Yoshihiro Ito 1, 2, 4 , Hideyuki Miyatake 1, 5
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2021-01-15 , DOI: 10.1021/acschembio.0c00817 Boyang Ning 1, 2 , Xueli Ren 1 , Kyoji Hagiwara 1 , Shinji Takeoka 1, 2, 3 , Yoshihiro Ito 1, 2, 4 , Hideyuki Miyatake 1, 5
Affiliation
|
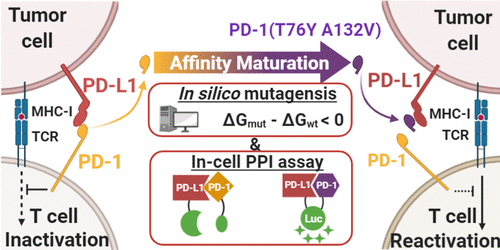
Inhibiting the programmed death-1 (PD-1)/programmed death ligand 1 (PD-L1) axis by monoclonal antibodies (mAbs) is a successful cancer immunotherapy. However, mAb-based drugs have various disadvantages including high production costs and large molecular sizes, which motivated us to develop a smaller alternative drug. Since PD-L1 binds PD-1 with moderate affinity, a higher affinity PD-1 variant should serve as a competitive inhibitor of the wild-type PD-1/PD-L1 interaction. In this report, we conducted in silico point mutagenesis of PD-1 to identify potent PD-1 variants with a higher affinity toward PD-L1 and refined the in silico results using a luciferase-based in-cell protein–protein interaction (PPI) assay. As a result, a PD-1 variant was developed that had two mutated amino acids (T76Y, A132V), termed 2-PD-1. 2-PD-1 could bind with PD-L1 at a dissociation constant of 12.74 nM. Moreover, 2-PD-1 successfully inhibited the PD-1/PD-L1 interaction with a half maximal inhibitory concentration of 19.15 nM and reactivated the T cell with a half maximal effective concentration of 136.1 nM. These results show that in silico mutagenesis combined with an in-cell PPI assay verification strategy successfully prepared a non-IgG inhibitor of the PD-1/PD-L1 interaction.
中文翻译:

通过计算机诱变和细胞内蛋白质-蛋白质相互作用测定开发非IgG PD-1 / PD-L1抑制剂
通过单克隆抗体(mAb)抑制程序性死亡1(PD-1)/程序性死亡配体1(PD-L1)轴是一种成功的癌症免疫疗法。但是,基于mAb的药物具有多种缺点,包括生产成本高和分子大小大,这促使我们开发出较小的替代药物。由于PD-L1以中等亲和力与PD-1结合,因此亲和力更高的PD-1变体应作为野生型PD-1 / PD-L1相互作用的竞争性抑制剂。在本报告中,我们进行了PD-1的计算机硅点诱变,以鉴定对PD-L1具有更高亲和力的有效PD-1变体,并改进了计算机使用基于萤光素酶的细胞内蛋白质-蛋白质相互作用(PPI)分析的结果。结果,开发了PD-1变体,它具有两个突变的氨基酸(T76Y,A132V),称为2-PD-1。2-PD-1可以以12.74 nM的解离常数与PD-L1结合。此外,2-PD-1以最大抑制浓度的一半为19.15 nM成功抑制了PD-1 / PD-L1的相互作用,并以最大有效浓度的一半为136.1 nM重新激活了T细胞。这些结果表明,计算机诱变结合细胞内PPI分析验证策略成功制备了PD-1 / PD-L1相互作用的非IgG抑制剂。
更新日期:2021-02-19
中文翻译:

通过计算机诱变和细胞内蛋白质-蛋白质相互作用测定开发非IgG PD-1 / PD-L1抑制剂
通过单克隆抗体(mAb)抑制程序性死亡1(PD-1)/程序性死亡配体1(PD-L1)轴是一种成功的癌症免疫疗法。但是,基于mAb的药物具有多种缺点,包括生产成本高和分子大小大,这促使我们开发出较小的替代药物。由于PD-L1以中等亲和力与PD-1结合,因此亲和力更高的PD-1变体应作为野生型PD-1 / PD-L1相互作用的竞争性抑制剂。在本报告中,我们进行了PD-1的计算机硅点诱变,以鉴定对PD-L1具有更高亲和力的有效PD-1变体,并改进了计算机使用基于萤光素酶的细胞内蛋白质-蛋白质相互作用(PPI)分析的结果。结果,开发了PD-1变体,它具有两个突变的氨基酸(T76Y,A132V),称为2-PD-1。2-PD-1可以以12.74 nM的解离常数与PD-L1结合。此外,2-PD-1以最大抑制浓度的一半为19.15 nM成功抑制了PD-1 / PD-L1的相互作用,并以最大有效浓度的一半为136.1 nM重新激活了T细胞。这些结果表明,计算机诱变结合细胞内PPI分析验证策略成功制备了PD-1 / PD-L1相互作用的非IgG抑制剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号