当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Annulative Methods in the Synthesis of Complex Meroterpene Natural Products
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-01-15 , DOI: 10.1021/acs.accounts.0c00781 Xingyu Shen 1 , Danny Q Thach 1 , Chi P Ting 1, 2 , Thomas J Maimone 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-01-15 , DOI: 10.1021/acs.accounts.0c00781 Xingyu Shen 1 , Danny Q Thach 1 , Chi P Ting 1, 2 , Thomas J Maimone 1
Affiliation
|
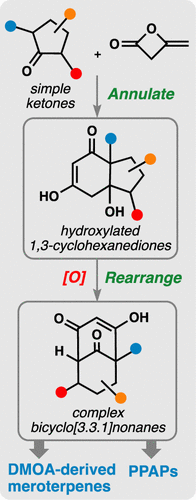
From the venerable Robinson annulation to the irreplaceable Diels–Alder cycloaddition, annulation reactions have fueled the progression of the field of natural product synthesis throughout the past century. In broader terms, the ability to form a cyclic molecule directly from two or more simpler fragments has transformed virtually every aspect of the chemical sciences from the synthesis of organic materials to bioconjugation chemistry and drug discovery. In this Account, we describe the evolution of our meroterpene synthetic program over the past five years, enabled largely by the development of a tailored anionic annulation process for the synthesis of hydroxylated 1,3-cyclohexanediones from lithium enolates and the reactive β-lactone-containing feedstock chemical diketene.
中文翻译:

复杂的美罗萜天然产物合成中的环形方法
从古老的Robinson环空法到不可替代的Diels-Alder环加成法,整个过去的世纪中,环空反应推动了天然产物合成领域的发展。从广义上讲,直接由两个或更多个简单片段形成环状分子的能力实际上已改变了化学科学的各个方面,从有机材料的合成到生物共轭化学和药物发现。在此帐户中,我们将描述过去五年中美特萜合成计划的发展,这主要是通过开发定制的阴离子环化工艺来实现的,该工艺可从烯醇锂和反应性β-内酯-合成羟基化1,3-环己二酮含有原料化学双烯酮。
更新日期:2021-02-02
中文翻译:

复杂的美罗萜天然产物合成中的环形方法
从古老的Robinson环空法到不可替代的Diels-Alder环加成法,整个过去的世纪中,环空反应推动了天然产物合成领域的发展。从广义上讲,直接由两个或更多个简单片段形成环状分子的能力实际上已改变了化学科学的各个方面,从有机材料的合成到生物共轭化学和药物发现。在此帐户中,我们将描述过去五年中美特萜合成计划的发展,这主要是通过开发定制的阴离子环化工艺来实现的,该工艺可从烯醇锂和反应性β-内酯-合成羟基化1,3-环己二酮含有原料化学双烯酮。









































 京公网安备 11010802027423号
京公网安备 11010802027423号