当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual role of a (p)ppGpp- and (p)ppApp-degrading enzyme in biofilm formation and interbacterial antagonism
Molecular Microbiology ( IF 2.6 ) Pub Date : 2021-01-15 , DOI: 10.1111/mmi.14684 Wieland Steinchen 1 , Shehryar Ahmad 2, 3 , Martina Valentini 4, 5 , Kira Eilers 5 , Mohamad Majkini 1 , Florian Altegoer 1 , Marcus Lechner 1 , Alain Filloux 5 , John C Whitney 2, 3, 6 , Gert Bange 1
Molecular Microbiology ( IF 2.6 ) Pub Date : 2021-01-15 , DOI: 10.1111/mmi.14684 Wieland Steinchen 1 , Shehryar Ahmad 2, 3 , Martina Valentini 4, 5 , Kira Eilers 5 , Mohamad Majkini 1 , Florian Altegoer 1 , Marcus Lechner 1 , Alain Filloux 5 , John C Whitney 2, 3, 6 , Gert Bange 1
Affiliation
|
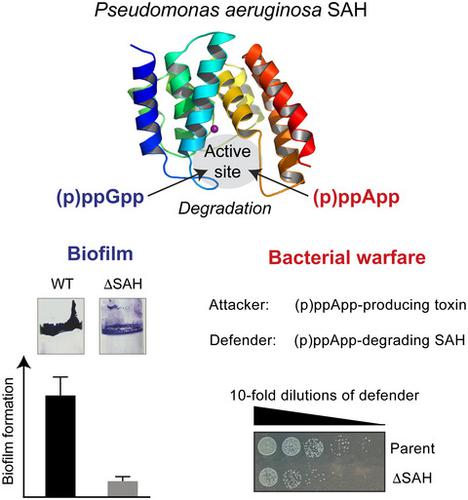
The guanosine nucleotide-based second messengers ppGpp and pppGpp (collectively: (p)ppGpp) enable adaptation of microorganisms to environmental changes and stress conditions. In contrast, the closely related adenosine nucleotides (p)ppApp are involved in type VI secretion system (T6SS)-mediated killing during bacterial competition. Long RelA-SpoT Homolog (RSH) enzymes regulate synthesis and degradation of (p)ppGpp (and potentially also (p)ppApp) through their synthetase and hydrolase domains, respectively. Small alarmone hydrolases (SAH) that consist of only a hydrolase domain are found in a variety of bacterial species, including the opportunistic human pathogen Pseudomonas aeruginosa. Here, we present the structure and mechanism of P. aeruginosa SAH showing that the enzyme promiscuously hydrolyses (p)ppGpp and (p)ppApp in a strictly manganese-dependent manner. While being dispensable for P. aeruginosa growth or swimming, swarming, and twitching motilities, its enzymatic activity is required for biofilm formation. Moreover, (p)ppApp-degradation by SAH provides protection against the T6SS (p)ppApp synthetase effector Tas1, suggesting that SAH enzymes can also serve as defense proteins during interbacterial competition.
中文翻译:

(p)ppGpp-和(p)ppApp-降解酶在生物膜形成和细菌间拮抗中的双重作用
基于鸟苷核苷酸的第二信使 ppGpp 和 pppGpp(统称:(p)ppGpp)使微生物能够适应环境变化和压力条件。相比之下,密切相关的腺苷核苷酸 (p)ppApp 在细菌竞争期间参与 VI 型分泌系统 (T6SS) 介导的杀伤。长- [R ELA-小号锅ħ omolog(RSH)酶通过它们的合成酶和水解酶结构域分别调节合成和(p)ppGpp的降解(并且潜在地也(P)ppApp)。小号商场一个larmone ħ ydrolases(SAH),其仅由水解酶结构域在多种细菌物种的发现,包括机会性人病原体铜绿假单胞菌。在这里,我们展示了铜绿假单胞菌SAH的结构和机制,表明该酶以严格依赖锰的方式混杂水解 (p)ppGpp 和 (p)ppApp。虽然对于铜绿假单胞菌的生长或游泳、蜂群和抽搐运动是可有可无的,但其酶活性是生物膜形成所必需的。此外,SAH 对 (p)ppApp 的降解提供了针对 T6SS (p)ppApp 合成酶效应子 Tas1 的保护,这表明 SAH 酶也可以在细菌间竞争期间充当防御蛋白。
更新日期:2021-01-15
中文翻译:

(p)ppGpp-和(p)ppApp-降解酶在生物膜形成和细菌间拮抗中的双重作用
基于鸟苷核苷酸的第二信使 ppGpp 和 pppGpp(统称:(p)ppGpp)使微生物能够适应环境变化和压力条件。相比之下,密切相关的腺苷核苷酸 (p)ppApp 在细菌竞争期间参与 VI 型分泌系统 (T6SS) 介导的杀伤。长- [R ELA-小号锅ħ omolog(RSH)酶通过它们的合成酶和水解酶结构域分别调节合成和(p)ppGpp的降解(并且潜在地也(P)ppApp)。小号商场一个larmone ħ ydrolases(SAH),其仅由水解酶结构域在多种细菌物种的发现,包括机会性人病原体铜绿假单胞菌。在这里,我们展示了铜绿假单胞菌SAH的结构和机制,表明该酶以严格依赖锰的方式混杂水解 (p)ppGpp 和 (p)ppApp。虽然对于铜绿假单胞菌的生长或游泳、蜂群和抽搐运动是可有可无的,但其酶活性是生物膜形成所必需的。此外,SAH 对 (p)ppApp 的降解提供了针对 T6SS (p)ppApp 合成酶效应子 Tas1 的保护,这表明 SAH 酶也可以在细菌间竞争期间充当防御蛋白。











































 京公网安备 11010802027423号
京公网安备 11010802027423号