Water Research ( IF 11.4 ) Pub Date : 2021-01-15 , DOI: 10.1016/j.watres.2021.116843 Yidan Gao 1 , Shifa Zhong 1 , Tifany L Torralba-Sanchez 2 , Paul G Tratnyek 2 , Eric J Weber 3 , Yiling Chen 4 , Huichun Zhang 1
|
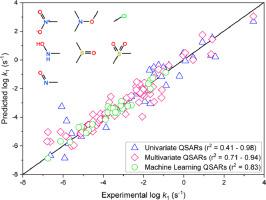
Due to the increasing diversity of organic contaminants discharged into anoxic water environments, reactivity prediction is necessary for chemical persistence evaluation for water treatment and risk assessment purposes. Almost all quantitative structure activity relationships (QSARs) that describe rates of contaminant transformation apply only to narrowly-defined, relatively homogenous families of reactants (e.g., dechlorination of alkyl halides). In this work, we develop predictive models for abiotic reduction of 60 organic compounds with diverse reducible functional groups, including nitroaromatic compounds (NACs), aliphatic nitro-compounds (ANCs), aromatic N-oxides (ANOs), isoxazoles (ISXs), polyhalogenated alkanes (PHAs), sulfoxides and sulfones (SOs), and others. Rate constants for their reduction were measured using a model reductant system, Fe(II)-tiron. Qualitatively, the rates followed the order NACs > ANOs ≈ ISXs ≈ PHAs > ANCs > SOs. To develop QSARs, both conventional chemical descriptor-based and machine learning (ML)-based approaches were investigated. Conventional univariate QSARs based on a molecular descriptor ELUMO (energy of the lowest-unoccupied molecular orbital) gave good correlations within classes. Multivariate QSARs combining ELUMO with Abraham descriptors for physico-chemical properties gave slightly improved correlations within classes for NCs and NACs, but little improvement in correlation within other classes or among classes. The ML model obtained covers reduction rates for all classes of compounds and all of the conditions studied with the prediction accuracy similar to those of the conventional QSARs for individual classes (r2 = 0.41–0.98 for univariate QSARs, 0.71–0.94 for multivariate QSARs, and 0.83 for the ML model). Both approaches required a scheme for a priori classification of the compounds for model training. This work offers two alternative modeling approaches to comprehensive abiotic reactivity prediction for persistence evaluation of organic compounds in anoxic water environments.
中文翻译:

水性 Fe(II) 配合物非生物还原有机化合物的定量结构活性关系 (QSAR) 和机器学习模型
由于排放到缺氧水环境中的有机污染物的多样性日益增加,反应性预测对于水处理和风险评估的化学持久性评估是必要的。几乎所有描述污染物转化率的定量构效关系 (QSAR) 仅适用于定义狭窄、相对同质的反应物族(例如卤代烷脱氯)。在这项工作中,我们开发了 60 种具有不同可还原官能团的有机化合物的非生物还原预测模型,包括硝基芳族化合物 (NAC)、脂肪族硝基化合物 (ANC)、芳香族 N-氧化物 (ANO)、异恶唑 (ISX)、多卤代烷烃 (PHA)、亚砜和砜 (SO) 等。使用模型还原剂系统测量它们的还原速率常数,Fe(II)-铁。定性地,比率遵循 NACs > ANOs ≈ ISXs ≈ PHAs > ANCs > SOs 的顺序。为了开发 QSAR,研究了基于常规化学描述符和基于机器学习 (ML) 的方法。基于分子描述符的传统单变量 QSARE LUMO(最低未占分子轨道的能量)在类内给出了良好的相关性。多变量 QSAR 将E LUMO与 Abraham 物理化学性质描述符相结合,NCs 和 NACs 类内的相关性略有改善,但其他类内或类间的相关性几乎没有改善。获得的 ML 模型涵盖了所有类别的化合物和所有研究条件的还原率,其预测精度与单个类别的常规 QSAR 相似(r 2 = 0.41–0.98(单变量 QSAR),0.71–0.94(多变量 QSAR)和 0.83(ML 模型)。这两种方法都需要一个用于模型训练的化合物的先验分类方案。这项工作为缺氧水环境中有机化合物的持久性评估提供了两种替代建模方法来综合非生物反应性预测。











































 京公网安备 11010802027423号
京公网安备 11010802027423号