Structure ( IF 4.4 ) Pub Date : 2021-01-15 , DOI: 10.1016/j.str.2020.12.009 Bronwyn J E Lyons 1 , Claire E Atkinson 2 , Wanyin Deng 3 , Antonio Serapio-Palacios 4 , B Brett Finlay 5 , Natalie C J Strynadka 2
|
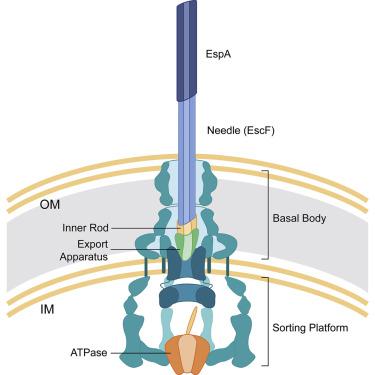
The type III secretion system (T3SS) is a virulence mechanism employed by Gram-negative pathogens. The T3SS forms a proteinaceous channel that projects a needle into the extracellular medium where it interacts with the host cell to deliver virulence factors. Enteropathogenic Escherichia coli (EPEC) is unique in adopting a needle extension to the T3SS—a filament formed by EspA—which is absolutely required for efficient colonization of the gut. Here, we describe the cryoelectron microscopy structure of native EspA filaments from EPEC at 3.6-Å resolution. Within the filament, positively charged residues adjacent to a hydrophobic groove line the lumen of the filament in a spiral manner, suggesting a mechanism of substrate translocation mediated via electrostatics. Using structure-guided mutagenesis, in vivo studies corroborate the role of these residues in secretion and translocation function. The high-resolution structure of the EspA filament could aid in structure-guided drug design of antivirulence therapeutics.
中文翻译:

来自肠致病性大肠杆菌的 EspA 细丝的冷冻电镜结构:揭示 T3SS 中效应子易位的机制
III 型分泌系统 (T3SS) 是革兰氏阴性病原体采用的毒力机制。T3SS 形成一个蛋白质通道,将针头投射到细胞外介质中,在那里它与宿主细胞相互作用以传递毒力因子。肠致病性大肠杆菌(EPEC) 的独特之处在于将针头延伸到 T3SS(一种由 EspA 形成的细丝),这是肠道有效定植所必需的。在这里,我们以 3.6 Å 的分辨率描述了来自 EPEC 的天然 EspA 细丝的冷冻电子显微镜结构。在灯丝内,与疏水凹槽相邻的带正电荷的残基以螺旋方式排列在灯丝的管腔内,表明存在通过静电介导的底物易位机制。使用结构引导的诱变,体内研究证实了这些残基在分泌和易位功能中的作用。EspA 灯丝的高分辨率结构有助于抗病毒治疗的结构引导药物设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号