Applied Clay Science ( IF 5.3 ) Pub Date : 2021-01-15 , DOI: 10.1016/j.clay.2020.105964 Xiaotong Yang , Yibo Gao , Zhenzhen Zhao , Ye Tian , Xianggui Kong , Xiaodong Lei , Fazhi Zhang
|
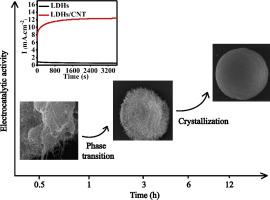
This study reports the facile fabrication of a three-dimensional (3D) spherical composite of NiII, FeIII-layered double hydroxides (NiFe-LDHs)/carbon nanotube (CNT) via the urea synthesis method without any surfactants or organic solvents and applies it to an ethanol electrooxidation reaction for the first time. The spherical composite showed a particle size of approximately 4 μm with NiFe-LDHs sheets that were closely arranged on the sphere surfaces. The formation mechanism of the composite was inferred. First, NiII and FeIII ions were adsorbed on the CNT surface. Simultaneously, the CNT agglomerated into interwoven clusters to form a conductive network. Sequential hydrolysis and polycondensation of FeIII in the presence of urea were kinetically beneficial to the precipitation of amorphous iron hydroxide. Then, an in situ phase transition from amorphous iron hydroxide to α-FeOOH/Fe(OH)3 nanoflakes occurred. Surface-adsorbed NiII was doped into the α-FeOOH/Fe(OH)3 flakes. Finally, the NiFe-LDHs nanoflakes continued to crystallize and formed a NiFe-LDHs/CNT spherical composite. Compared to the NiFe-LDHs powder sample, the NiFe-LDHs/CNT composite had enhanced electrocatalytic activity (approximately 5 times), showed a smaller electron transfer resistance of 15.7 Ω, and increased the long-term stability of ethanol oxidation, which could be deduced from the synergistic effect between NiFe-LDHs and CNT.
中文翻译:

层状双氢氧化物/碳纳米管的三维球形复合材料用于乙醇的电催化
这项研究报告了通过尿素合成方法,无需任何表面活性剂或有机溶剂,即可轻松地制备Ni II,Fe III层状双氢氧化物(NiFe-LDHs)/碳纳米管(CNT)的三维(3D)球形复合材料的方法,并将其应用于首次将其用于乙醇电氧化反应。球形复合材料的NiFe-LDHs片紧密排列在球体表面,粒径约为4μm。推测了复合材料的形成机理。首先,Ni II和Fe III离子被吸附在CNT表面上。同时,CNT聚集成交织的簇以形成导电网络。Fe III的顺序水解和缩聚在尿素的存在下对无定形氢氧化铁的沉淀在动力学上有利。然后,发生了从无定形氢氧化铁到α-FeOOH/ Fe(OH)3纳米薄片的原位相变。将表面吸附的Ni II掺杂到α-FeOOH/ Fe(OH)3薄片中。最后,NiFe-LDHs纳米片继续结晶并形成NiFe-LDHs / CNT球形复合材料。与NiFe-LDHs粉末样品相比,NiFe-LDHs / CNT复合材料具有增强的电催化活性(约5倍),显示出较小的15.7Ω电子传递阻力,并提高了乙醇氧化的长期稳定性,这可能是因为由NiFe-LDHs与CNT之间的协同作用推导得出。











































 京公网安备 11010802027423号
京公网安备 11010802027423号