当前位置:
X-MOL 学术
›
J. Mater. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A novel cyanoacrylate-based matrix excipient in HPMCP capsules forms a sustained intestinal delivery system for orally administered drugs with enhanced absorption efficiency
Journal of Materials Chemistry B ( IF 7 ) Pub Date : 2020-12-22 , DOI: 10.1039/d0tb02606a Liya Song 1, 2, 3, 4, 5 , Pengfei Chen 4, 6, 7, 8, 9 , Jin Yu 4, 8, 9, 10 , Xiaolu Han 1, 2, 3, 4 , Yabing Hua 1, 2, 3, 4 , Shan Liu 4, 11, 12 , Bo Pang 4, 13, 14, 15, 16 , Jing Gao 1, 2, 3, 4 , Jiahua Ma 4, 5, 17, 18 , Liang Xu 1, 2, 3, 4
Journal of Materials Chemistry B ( IF 7 ) Pub Date : 2020-12-22 , DOI: 10.1039/d0tb02606a Liya Song 1, 2, 3, 4, 5 , Pengfei Chen 4, 6, 7, 8, 9 , Jin Yu 4, 8, 9, 10 , Xiaolu Han 1, 2, 3, 4 , Yabing Hua 1, 2, 3, 4 , Shan Liu 4, 11, 12 , Bo Pang 4, 13, 14, 15, 16 , Jing Gao 1, 2, 3, 4 , Jiahua Ma 4, 5, 17, 18 , Liang Xu 1, 2, 3, 4
Affiliation
|
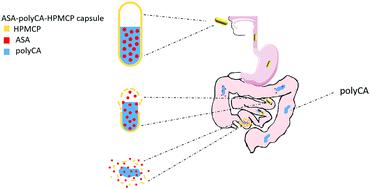
Patients prefer oral drug delivery due to its convenience and noninvasiveness. Nevertheless, a multitude of potentially clinically important drugs will not reach the market or achieve their full potential, due to their low bioavailability and instability in gastric acid. In this study, a novel oral drug delivery system based on poly-cyanoacrylate [a polymer of 2-(2-methoxyethoxy)ethyl-2-cyanoacrylate (MECA)] and hydroxypropyl methylcellulose phthalate (HPMCP) was developed and shown to permit intestinal targeting and sustained drug release. Aspirin [acetylsalicylic acid (ASA)] was selected as a model drug for atherosclerosis treatment. It was physically dissolved in liquid MECA, and the ASA–MECA matrix was then polymerized into a solid drug-loading depot in an HPMCP shell. The delivery of the drug depot in the intestine was achieved with the HPMCP shell; then the polymerized MECA (polyMECA) provided sustained drug release. The polyMECA excipient was not absorbed by the intestine due to its high molecular weight; a fluorescein-labeled assay indicated that it was excreted completely in feces after drug release. The formulation, ASA–polyMECA–HPMCP, showed good intestinal targeting and sustained drug release in vitro and in vivo. Pharmacokinetic studies indicated that this formulation improved the bioavailability of ASA relative to commercially available controls. ASA–polyMECA–HPMCP showed desirable anti-atherosclerosis efficacy in a rabbit model, with significant enhancement of atheromatous lesion stability. Biosafety tests proved the low toxicity of ASA–polyMECA–HPMCP and the polyMECA matrix. We believe that this work has provided a practical and biocompatible system for sustained intestinal drug delivery that can be applied broadly with various drugs for specific therapeutic aims.
中文翻译:

HPMCP胶囊中基于氰基丙烯酸酯的新型基质赋形剂形成了口服给药的持续肠道输送系统,具有增强的吸收效率
由于其方便和无创性,患者更喜欢口服药物递送。然而,由于它们的低生物利用度和胃酸的不稳定性,许多具有临床意义的潜在重要药物将无法上市或发挥其全部潜力。在这项研究中,开发了一种基于聚氰基丙烯酸酯[2-(2-甲氧基乙氧基)乙基-2-氰基丙烯酸酯(MECA)的聚合物]和羟丙基甲基纤维素邻苯二甲酸酯(HPMCP)的新型口服药物递送系统,并证明可实现肠道靶向和持续的药物释放。选择阿司匹林[乙酰水杨酸(ASA)]作为治疗动脉粥样硬化的模型药物。将其物理溶解在液态MECA中,然后将ASA–MECA基质聚合到HPMCP壳中的固体药物装载库中。HPMCP外壳可实现药物在肠道中的递送;然后聚合的MECA(polyMECA)提供了持续的药物释放。聚MECA赋形剂因分子量高而未被肠吸收。荧光素标记的测定表明药物释放后,它已完全排泄在粪便中。ASA–polyMECA–HPMCP制剂显示出良好的肠道靶向性和持续的药物释放体外和体内。药代动力学研究表明,相对于市售对照,该制剂改善了ASA的生物利用度。ASA–polyMECA–HPMCP在兔模型中显示出理想的抗动脉粥样硬化功效,并显着增强了动脉粥样硬化病变的稳定性。生物安全性测试证明了ASA–polyMECA–HPMCP和polyMECA基质的低毒性。我们相信这项工作为持续肠道药物的输送提供了一种实用且生物相容的系统,该系统可以与各种药物广泛地用于特定的治疗目的。
更新日期:2021-01-14
中文翻译:

HPMCP胶囊中基于氰基丙烯酸酯的新型基质赋形剂形成了口服给药的持续肠道输送系统,具有增强的吸收效率
由于其方便和无创性,患者更喜欢口服药物递送。然而,由于它们的低生物利用度和胃酸的不稳定性,许多具有临床意义的潜在重要药物将无法上市或发挥其全部潜力。在这项研究中,开发了一种基于聚氰基丙烯酸酯[2-(2-甲氧基乙氧基)乙基-2-氰基丙烯酸酯(MECA)的聚合物]和羟丙基甲基纤维素邻苯二甲酸酯(HPMCP)的新型口服药物递送系统,并证明可实现肠道靶向和持续的药物释放。选择阿司匹林[乙酰水杨酸(ASA)]作为治疗动脉粥样硬化的模型药物。将其物理溶解在液态MECA中,然后将ASA–MECA基质聚合到HPMCP壳中的固体药物装载库中。HPMCP外壳可实现药物在肠道中的递送;然后聚合的MECA(polyMECA)提供了持续的药物释放。聚MECA赋形剂因分子量高而未被肠吸收。荧光素标记的测定表明药物释放后,它已完全排泄在粪便中。ASA–polyMECA–HPMCP制剂显示出良好的肠道靶向性和持续的药物释放体外和体内。药代动力学研究表明,相对于市售对照,该制剂改善了ASA的生物利用度。ASA–polyMECA–HPMCP在兔模型中显示出理想的抗动脉粥样硬化功效,并显着增强了动脉粥样硬化病变的稳定性。生物安全性测试证明了ASA–polyMECA–HPMCP和polyMECA基质的低毒性。我们相信这项工作为持续肠道药物的输送提供了一种实用且生物相容的系统,该系统可以与各种药物广泛地用于特定的治疗目的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号