当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regioselective Synthesis of 2,3‐Dihydrobenzo[f]isoindolones via Ag‐Catalyzed Sequential Ugi 4CR/Cascade Radical Cyclization Reaction
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-01-13 , DOI: 10.1002/adsc.202001255 Ying‐Chun He 1 , Yan‐Mei Yan 2 , Zhen‐Xing Ren 1 , Yong‐Zhao Wang 3 , Qiang Yu 4 , Jun Xiong 5 , Meng‐Liang Wang 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-01-13 , DOI: 10.1002/adsc.202001255 Ying‐Chun He 1 , Yan‐Mei Yan 2 , Zhen‐Xing Ren 1 , Yong‐Zhao Wang 3 , Qiang Yu 4 , Jun Xiong 5 , Meng‐Liang Wang 1
Affiliation
|
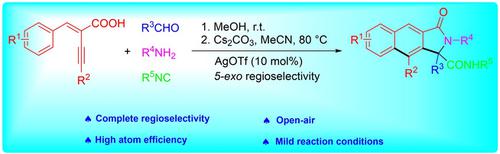
An approach to 2,3‐dihydrobenzo[f]isoindolones by a Ag‐catalyzed Ugi 4CR/cascade radical cyclization sequence under mild conditions has been developed. The reaction of (E)‐2‐benzylidene‐4‐arylbut‐3‐ynoic acids, aldehydes, amines, and isocyanides produced 2,3‐dihydro‐benzo[f]isoindolones regioselectively in 60–85% yields via sequential Ugi 4CR/cascade radical cyclization reaction in the presence of Cs2CO3 and AgOTf. The method achieved the formation of two C−C bonds under air atmosphere in one‐pot fashion.
中文翻译:

Ag催化的连续Ugi 4CR /级联自由基环化反应区域选择性合成2,3-二氢苯并[f]异吲哚酮
已经开发了一种在温和条件下通过Ag催化的Ugi 4CR /级联自由基环化序列制备2,3-二氢苯并[ f ]异吲哚酮的方法。(E)-2-亚苄基-4-芳基丁-3-炔酸,醛,胺和异氰酸酯的反应通过连续的Ugi 4CR /选择性地产生60-85%的2,3-二氢苯并[ f ]异吲哚酮。 Cs 2 CO 3和AgOTf存在下进行级联自由基环化反应 该方法以一锅法在空气气氛下实现了两个C-C键的形成。
更新日期:2021-02-16
中文翻译:

Ag催化的连续Ugi 4CR /级联自由基环化反应区域选择性合成2,3-二氢苯并[f]异吲哚酮
已经开发了一种在温和条件下通过Ag催化的Ugi 4CR /级联自由基环化序列制备2,3-二氢苯并[ f ]异吲哚酮的方法。(E)-2-亚苄基-4-芳基丁-3-炔酸,醛,胺和异氰酸酯的反应通过连续的Ugi 4CR /选择性地产生60-85%的2,3-二氢苯并[ f ]异吲哚酮。 Cs 2 CO 3和AgOTf存在下进行级联自由基环化反应 该方法以一锅法在空气气氛下实现了两个C-C键的形成。









































 京公网安备 11010802027423号
京公网安备 11010802027423号