当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electro‐Oxidation of Titanium Carbide Nanoparticles in Aqueous Acid Creates TiC@TiO2 Core‐Shell Structures
ChemElectroChem ( IF 3.5 ) Pub Date : 2021-01-13 , DOI: 10.1002/celc.202001498 Richard G. Compton 1 , Pranati Nayak 2 , Ruo-Chen Xie 2 , Robert G. Palgrave 3
ChemElectroChem ( IF 3.5 ) Pub Date : 2021-01-13 , DOI: 10.1002/celc.202001498 Richard G. Compton 1 , Pranati Nayak 2 , Ruo-Chen Xie 2 , Robert G. Palgrave 3
Affiliation
|
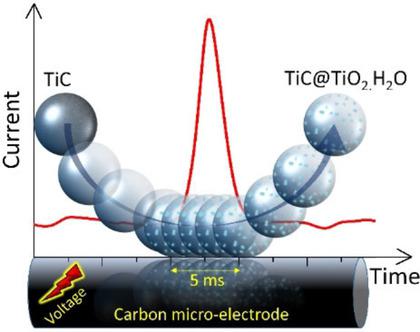
Titanium carbide (TiC) is an attractive support material used in electro‐catalysis and sensing. We report the electrochemistry of TiC nanoparticles (NPs, 35–50 nm in diameter) in different electrolytes in the pH range of 0 to 8. The TiC NPs undergo irreversible oxidation in acidic, basic, and neutral media, attributed to the partial conversion into titanium dioxide (TiO2) with the amount of oxidation highly dependent on the pH of the solution. In H2SO4 (pH 0), multiple voltammetric scans revealed the conversion to be partial but repeated scans allowed a conversion approaching 100 % to be obtained with 20 scans generating a ca 60 % level of oxidation. The process is inferred to lead to the formation of TiC@TiO2 core‐shell nanoparticles (∼12.5 nm core radius and ∼5 nm shell width for a 60 % conversion) and this value sharply decreases with an increase of pH. Independent measurements were conducted at a single NP level (via nano‐impact experiments) to confirm the oxidation of the NPs, showing consistent agreement with the bulk measurements.
中文翻译:

水溶液中碳化钛纳米粒子的电氧化产生TiC @ TiO2核壳结构
碳化钛(TiC)是用于电催化和传感的有吸引力的支撑材料。我们报告了在0至8的pH范围内,不同电解质中TiC纳米粒子(直径为35–50 nm的纳米粒子)的电化学反应。TiC纳米粒子在酸性,碱性和中性介质中经历不可逆的氧化,这归因于部分转化为氧化量与溶液的pH值高度相关的二氧化钛(TiO 2)。在H 2 SO 4(pH 0)中,多次伏安扫描显示该转化为部分转化,但重复扫描允许通过20次扫描产生接近60%的氧化水平,获得接近100%的转化率。推断该过程导致TiC @ TiO 2的形成核-壳纳米粒子(约12.5 nm的核半径和约5 nm的壳宽,可实现60%的转化),并且该值随着pH的增加而急剧下降。在单个NP水平上进行了独立测量(通过纳米碰撞实验),以确认NP的氧化,表明与批量测量结果一致。
更新日期:2021-03-01
中文翻译:

水溶液中碳化钛纳米粒子的电氧化产生TiC @ TiO2核壳结构
碳化钛(TiC)是用于电催化和传感的有吸引力的支撑材料。我们报告了在0至8的pH范围内,不同电解质中TiC纳米粒子(直径为35–50 nm的纳米粒子)的电化学反应。TiC纳米粒子在酸性,碱性和中性介质中经历不可逆的氧化,这归因于部分转化为氧化量与溶液的pH值高度相关的二氧化钛(TiO 2)。在H 2 SO 4(pH 0)中,多次伏安扫描显示该转化为部分转化,但重复扫描允许通过20次扫描产生接近60%的氧化水平,获得接近100%的转化率。推断该过程导致TiC @ TiO 2的形成核-壳纳米粒子(约12.5 nm的核半径和约5 nm的壳宽,可实现60%的转化),并且该值随着pH的增加而急剧下降。在单个NP水平上进行了独立测量(通过纳米碰撞实验),以确认NP的氧化,表明与批量测量结果一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号