Giant ( IF 5.4 ) Pub Date : 2021-01-14 , DOI: 10.1016/j.giant.2021.100047 Mineto Uchiyama , Kotaro Satoh , Masami Kamigaito
|
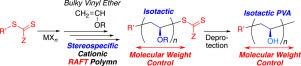
The simultaneous control of molecular weight and tacticity in cationic polymerization remains challenging. Here, we propose a new approach for stereospecific living cationic polymerization by combining cationic reversible addition-fragmentation chain transfer (RAFT) polymerization with thiocarbonylthio compounds and stereospecific cationic polymerization of bulky vinyl ethers with Lewis acid catalysts. In this combined system, the molecular weight was controlled by the RAFT process between the growing cationic and dormant dithiocarbamate chain ends, whereas the tacticity was controlled by stereospecific propagation based on the steric hindrance of bulky side groups and counteranions derived from the Lewis acid catalysts. Herein, we demonstrate the availability of this approach in the cationic polymerization of various bulky vinyl ethers, such as tert‑butyl, benzyl, trimethylsilyl, and tert‑butyldimethylsilyl vinyl ether, using dithiocarbamates and BF3•OEt2 or EtAlCl2 in toluene at –78 °C. Isotactic-rich poly(vinyl ether)s with controlled molecular weights and dithiocarbamate chain-end groups were obtained from a series of bulky vinyl ethers and were converted into molecular-weight-controlled isotactic poly(vinyl alcohol) (mm ≥ 70%) with a high melting temperature (Tm ~ 230 °C). Furthermore, isotactic-b-atactic stereoblock PVA (mm/mr/rr = 75/25/5 and 23/48/29, Tm ~ 210 °C) was synthesized by mechanistic transformation from the stereospecific cationic RAFT polymerization of trimethylsilyl vinyl ether into the radical RAFT polymerization of vinyl acetate using diphenyl dithiocarbamate as the common RAFT agent followed by simultaneous deprotection of the silyl and acetyl groups.
中文翻译:

机械转化为乙酸乙烯酯的自由基RAFT聚合反应的立体乙烯基阳离子RAFT聚合大体积乙烯基醚和立体嵌段聚乙烯醇
阳离子聚合中分子量和立构规整度的同时控制仍然具有挑战性。在这里,我们提出了一种新的立体定向活性阳离子聚合的方法,该方法将阳离子可逆加成-断裂链转移(RAFT)聚合与硫代羰基硫代化合物相结合,以及大体积乙烯基醚与路易斯酸催化剂的立体定向阳离子聚合。在该组合系统中,分子量由增长的阳离子和休眠二硫代氨基甲酸酯链末端之间的RAFT过程控制,而立构规整则基于庞大的侧基和衍生自Lewis酸催化剂的抗衡阴离子的空间位阻,通过立体定向传播来控制。本文中,我们证明了该方法在各种大体积乙烯基醚(例如叔丁基,苄基,三甲基甲硅烷基和叔丁基二甲基甲硅烷基乙烯基醚,使用二硫代氨基甲酸酯和BF 3 •OEt 2或EtAlCl 2在甲苯中的温度为–78°C。富全同立构的聚(乙烯基醚)具有受控的分子量和二硫代氨基甲酸的链末端基团从一系列笨重乙烯基醚的获得,并转化为分子量控制的全同立构的聚(乙烯醇)(毫米≥70%)与高熔融温度(Ť米〜230℃)。此外,等规-b-无规立体嵌段PVA(mm / mr / rr = 75/25/5和23 / 48 / 29,T m 通过将三甲基甲硅烷基乙烯基醚的立体定向阳离子RAFT聚合转化为乙酸乙烯酯的自由基RAFT聚合(使用二苯基二硫代氨基甲酸酯作为常见的RAFT剂),然后同时对甲硅烷基和乙酰基进行脱保护,通过机械转化合成了约210°C)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号