Surface & Coatings Technology ( IF 5.3 ) Pub Date : 2021-01-14 , DOI: 10.1016/j.surfcoat.2021.126840 Eren Seçkin , Mustafa Ürgen
|
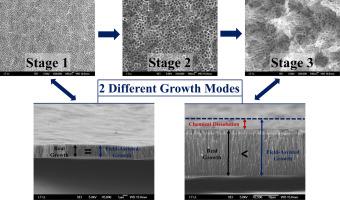
During porous anodization of titanium different surface morphologies can be observed. However, the influence of this morphological variance on nanotube growth has not been defined with a consistent model. In this study, we aim to develop a kinetic model-approach that combines the growth process with the final morphologies of the different nanotubular titania structures that are formed during anodization in ethylene glycol based electrolytes. Accordingly, we divide the nanotube growth process into three sequential stages. Morphological transitions and growth kinetics during anodization are investigated individually for all stages. In the first stage, nanotubes grow under gradually dissolving initial barrier oxide. Nanopore/nanotube transition occurs at the second stage after the complete dissolution of the initial barrier oxide. Nanograss formation starts and progresses at the third stage of the anodization. Growth of nanotubes under the gradually dissolving top barrier layer (Stage 1) obeyed the field-assisted growth model with a growth rate equivalent to 0.7 μm C−1 cm2. However, the completion of the chemical dissolution of top barrier layer totally changes the growth rate leading to a shortening of the nanotubes (Stage 3). After experimental determination of the chemical shortening rate of the nanotubes (CSR) at this stage, a kinetic model has been produced to determine the top barrier layer dissolution time (BDT). The experimental determination of the temperature dependency of BDT allowed us to calculate the activation energy of the process and determination of BDT values for different processing temperatures by extrapolation. Extrapolated BDT values for different temperatures showed well consistency with the experimental results and validate that open tube-top nanotubes can be obtained at calculated anodization durations.
中文翻译:

在乙二醇基电解质中钛阳极氧化过程中确定二氧化钛纳米管形态转变和生长动力学的动力学模型
在钛的多孔阳极氧化过程中,可以观察到不同的表面形态。但是,这种形态变化对纳米管生长的影响尚未用一致的模型定义。在这项研究中,我们旨在开发一种动力学模型方法,该方法将生长过程与在基于乙二醇的电解质中阳极氧化过程中形成的不同纳米管二氧化钛结构的最终形态相结合。因此,我们将纳米管的生长过程分为三个连续的阶段。阳极氧化过程中的各个阶段的形态学转变和生长动力学都进行了单独研究。在第一阶段,纳米管在逐渐溶解初始阻挡层氧化物的条件下生长。纳米孔/纳米管的转变发生在初始隔离氧化物完全溶解后的第二阶段。纳米草的形成在阳极氧化的第三阶段开始并进行。在逐渐溶解的顶部势垒层下(阶段1),纳米管的生长服从场辅助生长模型,其生长速率等于0.7μmC-1 cm 2。但是,顶部阻挡层化学溶解的完成完全改变了生长速率,导致纳米管缩短(阶段3)。在此阶段通过实验确定纳米管的化学缩短率(CSR)之后,已建立了动力学模型来确定顶部阻挡层溶解时间(BDT)。通过对BDT的温度依赖性进行实验确定,我们可以通过外推法来计算过程的活化能并确定不同处理温度下的BDT值。外推的BDT值在不同温度下显示出与实验结果良好的一致性,并证明可以在计算的阳极氧化持续时间下获得开放的管顶式纳米管。









































 京公网安备 11010802027423号
京公网安备 11010802027423号