Structure ( IF 5.7 ) Pub Date : 2021-01-14 , DOI: 10.1016/j.str.2020.12.007 Tatsuki Asai 1 , Naruhiko Adachi 2 , Toshio Moriya 2 , Hideyuki Oki 3 , Takamitsu Maru 3 , Masato Kawasaki 2 , Kano Suzuki 1 , Sisi Chen 1 , Ryohei Ishii 4 , Kazuko Yonemori 5 , Shigeru Igaki 5 , Satoshi Yasuda 6 , Satoshi Ogasawara 6 , Toshiya Senda 2 , Takeshi Murata 6
|
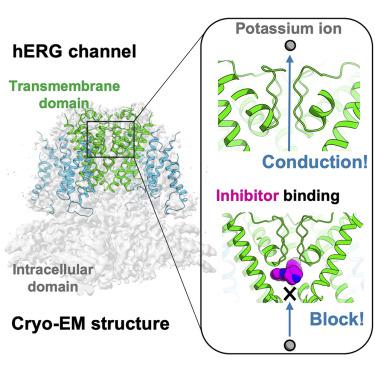
The hERG channel is a voltage-gated potassium channel involved in cardiac repolarization. Off-target hERG inhibition by drugs has become a critical issue in the pharmaceutical industry. The three-dimensional structure of the hERG channel was recently reported at 3.8-Å resolution using cryogenic electron microscopy (cryo-EM). However, the drug inhibition mechanism remains unclear because of the scarce structural information regarding the drug- and potassium-bound hERG channels. In this study, we obtained the cryo-EM density map of potassium-bound hERG channel complexed with astemizole, a well-known hERG inhibitor that increases risk of potentially fatal arrhythmia, at 3.5-Å resolution. The structure suggested that astemizole inhibits potassium conduction by binding directly below the selectivity filter. Furthermore, we propose a possible binding model of astemizole to the hERG channel and provide insights into the unusual sensitivity of hERG to several drugs.
中文翻译:

与阻滞剂阿司咪唑复合的 K+结合 hERG 通道的冷冻电镜结构
hERG 通道是参与心脏复极的电压门控钾通道。药物的脱靶 hERG 抑制已成为制药行业的一个关键问题。最近使用低温电子显微镜 (cryo-EM) 以 3.8 Å 的分辨率报道了 hERG 通道的三维结构。然而,药物抑制机制仍不清楚,因为关于药物和钾结合的 hERG 通道的结构信息很少。在这项研究中,我们获得了与阿司咪唑复合的钾结合 hERG 通道的冷冻电镜密度图,阿司咪唑是一种众所周知的 hERG 抑制剂,可增加潜在致命性心律失常的风险,分辨率为 3.5 Å。该结构表明阿司咪唑通过直接在选择性过滤器下方结合来抑制钾传导。此外,



























 京公网安备 11010802027423号
京公网安备 11010802027423号