Journal of Water Process Engineering ( IF 6.3 ) Pub Date : 2021-01-14 , DOI: 10.1016/j.jwpe.2020.101911 Golnoosh Khajouei , Hoil Il Park , Harry O. Finklea , Paul F. Ziemkiewicz , Edward F. Peltier , Lian-Shin Lin
|
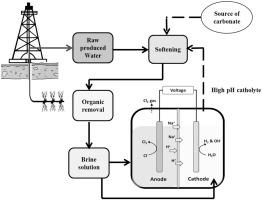
This study evaluates the benefits of using brine electrolysis for generating caustic soda (NaOH) and free chlorine for on-site produced water (PWs1) treatment. A two-compartment electrochemical cell was shown to generate NaOH solutions (pH > 12, faradic efficiency 93%) and chlorine (faradic efficiency 32%) from a NaCl brine solution at a current density of 10 mA/cm2. The catholyte was used for softening field-collected PWs. The degree of Mg removal depends mostly on the catholyte/PW mixture pH with pH 11 achieving >90% removal. Ca removal is poor (<10%) due to low bicarbonate alkalinity in the PWs. Soda ash alone at a dose equivalent to the total hardness of the PWs helps CaCO3 precipitation and Ca removal (>90%). The combined treatment of the catholyte and a reduced quantity of soda ash achieves better or comparable Ca removal compared to the full stoichiometric amounts of soda ash alone. Ba and Sr removal patterns closely follow those of Ca, suggesting co-precipitation of these cations as the primary removal mechanism. Organic removal is negligible during chemical softening; however, activated carbon filtration achieves >90% of total organic carbon (TOC) removal in all PWs. A treatment scheme is proposed for field generation of caustic soda and chlorine from PW. The economic analysis demonstrates the significant cost-effectiveness of the approach compared to purchasing the NaOH.
中文翻译:

使用盐水电解产生的高pH阴极电解液生产的水软化:减少化学物质的运输和环境足迹
这项研究评估了使用盐水电解产生苛性钠(NaOH)和游离氯进行现场采出水(PWs 1)处理的好处。已显示两室电化学电池以10 mA / cm 2的电流密度从NaCl盐水溶液生成NaOH溶液(pH> 12,法拉第效率为93%)和氯(法拉第效率为32%)。阴极电解液用于软化现场收集的PW。Mg的去除程度主要取决于阴极液/ PW混合物的pH值,其中pH 11达到> 90%的去除率。由于PW中碳酸氢盐的碱度低,因此Ca去除效果不佳(<10%)。纯碱的剂量等于PW的总硬度,有助于CaCO 3沉淀和除Ca(> 90%)。与仅化学计量的纯碱相比,对阴极电解液的处理和减少量的纯碱的组合处理可实现更好或相当的Ca去除率。Ba和Sr的去除模式紧随Ca的去除模式,表明这些阳离子的共沉淀是主要的去除机理。化学软化过程中有机物的去除可以忽略不计;但是,在所有PW中,活性炭过滤可达到> 90%的总有机碳(TOC)去除率。提出了一种处理方案,用于从PW现场生成苛性钠和氯。经济分析表明,与购买NaOH相比,该方法具有显着的成本效益。











































 京公网安备 11010802027423号
京公网安备 11010802027423号