当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mn(III)–porphyrin catalysts for the cycloaddition of CO2 with epoxides at atmospheric pressure: effects of Lewis acidity and ligand structure
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2021-1-4 , DOI: 10.1039/d0nj05280a Bruno Noschang Cabral 1, 2, 3, 4 , Jorge Luiz Sônego Milani 4, 5, 6, 7, 8 , Alexandre Moreira Meireles 4, 5, 6, 9, 10 , Dayse Carvalho da Silva Martins 4, 5, 6, 9, 10 , Stephany Larissa da Silva Ribeiro 1, 2, 3, 4 , Júlio Santos Rebouças 4, 5, 11, 12, 13 , Claudio Luis Donnici 4, 5, 6, 9, 10 , Rafael Pavão das Chagas 1, 2, 3, 4
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2021-1-4 , DOI: 10.1039/d0nj05280a Bruno Noschang Cabral 1, 2, 3, 4 , Jorge Luiz Sônego Milani 4, 5, 6, 7, 8 , Alexandre Moreira Meireles 4, 5, 6, 9, 10 , Dayse Carvalho da Silva Martins 4, 5, 6, 9, 10 , Stephany Larissa da Silva Ribeiro 1, 2, 3, 4 , Júlio Santos Rebouças 4, 5, 11, 12, 13 , Claudio Luis Donnici 4, 5, 6, 9, 10 , Rafael Pavão das Chagas 1, 2, 3, 4
Affiliation
|
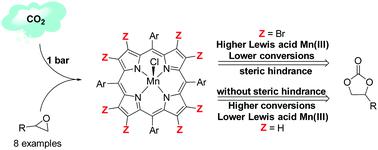
A series of eight Mn(III)–porphyrin (MnP) complexes with electron-withdrawing substituents at the meso and/or β-pyrrole positions of the macrocycle was designed to uncover electronic and structural aspects of MnP catalytic activity in the cycloaddition of CO2 with epoxides. The complexes, when combined with tetrabutylammonium halides, were active catalysts producing the respective cyclic carbonate under mild conditions. The non-β-brominated complex H3[MnT4CPP] served as a structural framework for the design of a series of homologous complexes, leading to the synthesis of the new β-brominated catalysts H3[Mn(BrxT4CPP)] (x = 2, 4, or 6). The β-brominated catalyst series allowed the investigation of the influence of structural effects versus electronic effects on the catalytic system, demonstrating a good correlation between the catalytic activity and the number of bromine substituents at the β-pyrrole positions. The non-planar distortions of the macrocycle and the consequent steric hindrance are determinant for the reaction outcome. The decrease in catalytic activity despite the increase in Lewis acidity of the metal center highlighted the effect of the out-of-plane distortion on the catalytic activity of manganese porphyrins.
中文翻译:

Mn(III)-卟啉催化剂,用于在大气压力下将环氧化物与CO2环加成:路易斯酸度和配体结构的影响
设计了一系列八个在大环的内消旋和/或β-吡咯位置带有吸电子取代基的Mn(III)-卟啉(MnP)配合物,以揭示CO 2环加成中MnP催化活性的电子和结构方面。与环氧化物。当与卤化四丁基铵结合时,该配合物是在温和条件下产生相应环状碳酸酯的活性催化剂。非β-溴化的配合物H 3 [MnT4CPP]作为一系列同源配合物设计的结构框架,导致合成了新的β-溴化的催化剂H 3 [Mn(Br x T4CPP)](x= 2、4或6)。β-溴化催化剂系列允许研究结构效应与电子效应对催化体系的影响,证明了催化活性与β-吡咯位置的溴取代基数量之间具有良好的相关性。大环的非平面畸变和随之而来的位阻是反应结果的决定因素。尽管金属中心的路易斯酸度增加了,但催化活性却降低了,这突出了面外形变对锰卟啉催化活性的影响。
更新日期:2021-01-13
中文翻译:

Mn(III)-卟啉催化剂,用于在大气压力下将环氧化物与CO2环加成:路易斯酸度和配体结构的影响
设计了一系列八个在大环的内消旋和/或β-吡咯位置带有吸电子取代基的Mn(III)-卟啉(MnP)配合物,以揭示CO 2环加成中MnP催化活性的电子和结构方面。与环氧化物。当与卤化四丁基铵结合时,该配合物是在温和条件下产生相应环状碳酸酯的活性催化剂。非β-溴化的配合物H 3 [MnT4CPP]作为一系列同源配合物设计的结构框架,导致合成了新的β-溴化的催化剂H 3 [Mn(Br x T4CPP)](x= 2、4或6)。β-溴化催化剂系列允许研究结构效应与电子效应对催化体系的影响,证明了催化活性与β-吡咯位置的溴取代基数量之间具有良好的相关性。大环的非平面畸变和随之而来的位阻是反应结果的决定因素。尽管金属中心的路易斯酸度增加了,但催化活性却降低了,这突出了面外形变对锰卟啉催化活性的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号