当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced analytical and physical characterization of mixtures of random bay-position brominated boron subnaphthalocyanines enabled by establishing a partial separation method
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2020-12-8 , DOI: 10.1039/d0nj04974c Devon P. Holst 1, 2, 3, 4 , Aleksa Dovijarski 1, 2, 3, 4 , Alan J. Lough 1, 2, 3, 4 , Timothy P. Bender 1, 2, 3, 4, 5
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2020-12-8 , DOI: 10.1039/d0nj04974c Devon P. Holst 1, 2, 3, 4 , Aleksa Dovijarski 1, 2, 3, 4 , Alan J. Lough 1, 2, 3, 4 , Timothy P. Bender 1, 2, 3, 4, 5
Affiliation
|
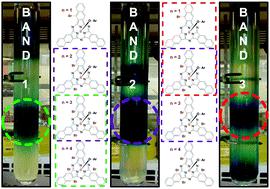
The synthesis of axially brominated boron subnaphthalocyanine (BsubNc) was investigated using BBr3 as the Lewis acid source. Random bay position bromination was found to occur as previously described by our group during the synthesis of chloro-(chloro)n-boron subnaphthalocyanines (Cl-ClnBsubNcs) using BCl3. The direct substitution of BBr3 for BCl3 in the synthesis of BsubNc was found to increase bay position halogenation. We found in this study that it is possible to avoid bay position bromination by employing low temperature synthetic methods. However, these methods were found to yield only analytical amounts of the BsubNc macrocycles, preventing any isolation and physical characterization. We identify herein an appropriate method for scaling of the synthesis of bromo-(bromo)n-boron subnaphthalocyanines (Br-BrnBsubNcs). On the appropriate scaling of the synthesis of bromo-(bromo)n-boron subnaphthalocyanines (Br-BrnBsubNcs), we then conducted axial phenoxylation (ArO-BrnBsubNcs) reactions to enable the separation of the mixture of bay position brominated species by column chromatography. Partial separation was accomplished. Separation and/or purification of Br-BrnBsubNcs is problematic given the known weakness of the B–Br bond which is widely open for hydrolysis. After partial separation, we describe herein analytical methods for the characterization of bay position halogenated BsubNcs with improved resolution and accuracy. These analytical techniques enable the accurate determination of the degrees of bay position halogenation that are present within a mixture of BsubNcs. Quantification of the average bay position halogenation and a description of the halogenated benz(f)isoindoline units within a mixture of bay position BsubNcs is also presented. The photophysical and electrochemical properties of the partially separated ArO-BrnBsubNcs were investigated. Bay position bromination was found to significantly influence these properties. A diminishing influence of bromination as the degree of bromination increased was observed in all cases. Fluorescence quantum yields of ArO-BrnBsubNcs were found to be close to an order of magnitude lower than those of their bay position chlorinated counterparts, presumably due to the heavy atom effect. Solution stability was also investigated, and a degradation product was identified by single-crystal X-ray diffraction analysis.
中文翻译:

通过建立部分分离方法,可以增强对无规海湾位置溴化亚萘基硼化硼混合物的分析和物理表征
使用BBr 3作为路易斯酸源研究了轴向溴化亚萘酞菁硼(BsubNc)的合成。如前所述,我们的小组在使用BCl 3合成氯-(氯)n-硼亚萘酞菁(Cl-Cl n BsubNcs)的过程中发现了随机的海湾位置溴化反应。用BBr 3直接代替BCl 3在BsubNc的合成中,发现增加海湾位置的卤化。我们在这项研究中发现,通过采用低温合成方法可以避免海湾位置溴化。但是,发现这些方法仅产生分析量的BsubNc大环,从而阻止了任何隔离和物理表征。我们在本文中确定了用于规模化溴-(溴)n-硼亚萘酞菁(Br-Br n BsubNcs)合成的合适方法。在适当规模的溴-(溴)n-硼亚萘酞菁(Br-Br n BsubNcs)的合成上,我们进行了轴向苯氧基化(ArO-Br nBsubNcs)反应,可通过柱色谱分离海湾位置溴化物种的混合物。部分分离完成。鉴于已知的B-Br键弱点已广泛开放,Br-Br n BsubNcs的分离和/或纯化存在问题。在部分分离之后,我们在此描述了具有改进的分辨率和精度的表征海湾位置卤代BsubNcs的分析方法。这些分析技术能够准确确定BsubNcs混合物中存在的海湾位置卤化度。平均海湾位置卤化的定量和卤代苯的描述(f还提出了在海湾位置BsubNcs的混合物中的异吲哚啉单元。研究了部分分离的ArO-Br n BsubNcs的光物理和电化学性质。发现海湾位置溴化显着影响这些性质。在所有情况下,随着溴化程度的增加,溴化的影响逐渐减小。发现ArO-Br n BsubNcs的荧光量子产率比其海湾位置氯化对等物的荧光量子产率低约一个数量级,这可能是由于重原子效应所致。还研究了溶液稳定性,并通过单晶X射线衍射分析鉴定了降解产物。
更新日期:2021-01-13
中文翻译:

通过建立部分分离方法,可以增强对无规海湾位置溴化亚萘基硼化硼混合物的分析和物理表征
使用BBr 3作为路易斯酸源研究了轴向溴化亚萘酞菁硼(BsubNc)的合成。如前所述,我们的小组在使用BCl 3合成氯-(氯)n-硼亚萘酞菁(Cl-Cl n BsubNcs)的过程中发现了随机的海湾位置溴化反应。用BBr 3直接代替BCl 3在BsubNc的合成中,发现增加海湾位置的卤化。我们在这项研究中发现,通过采用低温合成方法可以避免海湾位置溴化。但是,发现这些方法仅产生分析量的BsubNc大环,从而阻止了任何隔离和物理表征。我们在本文中确定了用于规模化溴-(溴)n-硼亚萘酞菁(Br-Br n BsubNcs)合成的合适方法。在适当规模的溴-(溴)n-硼亚萘酞菁(Br-Br n BsubNcs)的合成上,我们进行了轴向苯氧基化(ArO-Br nBsubNcs)反应,可通过柱色谱分离海湾位置溴化物种的混合物。部分分离完成。鉴于已知的B-Br键弱点已广泛开放,Br-Br n BsubNcs的分离和/或纯化存在问题。在部分分离之后,我们在此描述了具有改进的分辨率和精度的表征海湾位置卤代BsubNcs的分析方法。这些分析技术能够准确确定BsubNcs混合物中存在的海湾位置卤化度。平均海湾位置卤化的定量和卤代苯的描述(f还提出了在海湾位置BsubNcs的混合物中的异吲哚啉单元。研究了部分分离的ArO-Br n BsubNcs的光物理和电化学性质。发现海湾位置溴化显着影响这些性质。在所有情况下,随着溴化程度的增加,溴化的影响逐渐减小。发现ArO-Br n BsubNcs的荧光量子产率比其海湾位置氯化对等物的荧光量子产率低约一个数量级,这可能是由于重原子效应所致。还研究了溶液稳定性,并通过单晶X射线衍射分析鉴定了降解产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号