当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Residue-Selective Protein C-Formylation via Sequential Difluoroalkylation-Hydrolysis
ACS Central Science ( IF 12.7 ) Pub Date : 2021-01-12 , DOI: 10.1021/acscentsci.0c01193 Mateusz Imiołek 1, 2 , Patrick G. Isenegger 1 , Wai-Lung Ng 1 , Aziz Khan 1 , Véronique Gouverneur 1 , Benjamin G. Davis 1, 2
ACS Central Science ( IF 12.7 ) Pub Date : 2021-01-12 , DOI: 10.1021/acscentsci.0c01193 Mateusz Imiołek 1, 2 , Patrick G. Isenegger 1 , Wai-Lung Ng 1 , Aziz Khan 1 , Véronique Gouverneur 1 , Benjamin G. Davis 1, 2
Affiliation
|
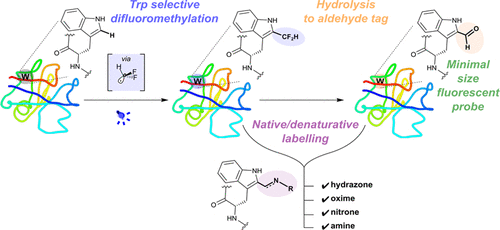
The carbonyl group is now a widely useful, nonproteinogenic functional group in chemical biology, yet methods for its generation in proteins have relied upon either cotranslational incorporation of unnatural amino acids bearing carbonyls or oxidative conversion (chemical or enzymatic) of existing natural amino acids. If available, alternative strategies for directly adding the C═O group through C–C bond-forming C-carbonylation, particularly at currently inaccessible amino acid sites, would provide a powerful method for adding valuable reactivity and expanding possible function in proteins. Here, following a survey of methods for HCF2· generation, we show that reductive photoredox catalysis enables mild radical-mediated difluoromethylation-hydrolysis of native protein residues as an effective method for carbonylation. Inherent selectivity of HCF2· allowed preferential modification of Trp residues. The resulting C-2-difluoromethylated Trp undergoes Reimer-Tiemann-type dehalogenation providing highly effective spontaneous hydrolytic collapse in proteins to carbonylated HC(O)-Trp (C-formyl-Trp = CfW) residues. This new, unnatural protein residue CfW not only was found to be effective in bioconjugation, ligation, and labeling reactions but also displayed strong “red-shifting” of its absorption and fluorescent emission maxima, allowing direct use of Trp sites as UV–visualized fluorophores in proteins and even cells. In this way, this method for the effective generation of masked formyl-radical “HC(O)·” equivalents enables first examples of C–C bond-forming carbonylation in proteins, thereby expanding the chemical reactivity and spectroscopic function that may be selectively and post-translationally “edited” into biology.
中文翻译:

通过顺序二氟烷基化-水解的残基选择性蛋白C-甲酰化
羰基现在是化学生物学中广泛使用的,不致蛋白质的官能团,但是在蛋白质中生成羰基的方法依赖于携带羰基的非天然氨基酸的共翻译结合或现有天然氨基酸的氧化转化(化学或酶促)。如果可以的话,通过形成C-C键的C-羰基直接加成C═O基团的替代策略,特别是在目前无法接近的氨基酸位点,将为增加有价值的反应性和扩展蛋白质的可能功能提供强大的方法。在这里,对HCF 2的方法进行了调查·生成,我们表明还原性光氧化还原催化能够使温和的自由基介导的天然蛋白质残基的二氟甲基化水解成为羰基化的有效方法。HCF 2 ·的固有选择性允许Trp残基的优先修饰。所得的C-2-二氟甲基化的Trp经历Reimer-Tiemann型脱卤作用,从而提供蛋白质中高效的自发水解塌陷成羰基化的HC(O)-Trp(C-甲酰基-Trp = CfW)残基。这种新的非天然蛋白残基CfW不仅在生物结合,连接和标记反应中有效,而且还显示出其吸收和荧光发射最大值的强烈“红移”,从而可以将Trp位点直接用作UV可视化的荧光团在蛋白质甚至细胞中。通过这种方式,这种有效生成掩蔽的甲酰基-自由基“ HC(O)·”等价物的方法使蛋白质中C–C键形成羰基化的第一个例子成为可能,从而扩大了化学反应性和光谱功能,这些选择性和选择性翻译后“编辑”为生物学。
更新日期:2021-01-27
中文翻译:

通过顺序二氟烷基化-水解的残基选择性蛋白C-甲酰化
羰基现在是化学生物学中广泛使用的,不致蛋白质的官能团,但是在蛋白质中生成羰基的方法依赖于携带羰基的非天然氨基酸的共翻译结合或现有天然氨基酸的氧化转化(化学或酶促)。如果可以的话,通过形成C-C键的C-羰基直接加成C═O基团的替代策略,特别是在目前无法接近的氨基酸位点,将为增加有价值的反应性和扩展蛋白质的可能功能提供强大的方法。在这里,对HCF 2的方法进行了调查·生成,我们表明还原性光氧化还原催化能够使温和的自由基介导的天然蛋白质残基的二氟甲基化水解成为羰基化的有效方法。HCF 2 ·的固有选择性允许Trp残基的优先修饰。所得的C-2-二氟甲基化的Trp经历Reimer-Tiemann型脱卤作用,从而提供蛋白质中高效的自发水解塌陷成羰基化的HC(O)-Trp(C-甲酰基-Trp = CfW)残基。这种新的非天然蛋白残基CfW不仅在生物结合,连接和标记反应中有效,而且还显示出其吸收和荧光发射最大值的强烈“红移”,从而可以将Trp位点直接用作UV可视化的荧光团在蛋白质甚至细胞中。通过这种方式,这种有效生成掩蔽的甲酰基-自由基“ HC(O)·”等价物的方法使蛋白质中C–C键形成羰基化的第一个例子成为可能,从而扩大了化学反应性和光谱功能,这些选择性和选择性翻译后“编辑”为生物学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号