Water Research ( IF 11.4 ) Pub Date : 2021-01-13 , DOI: 10.1016/j.watres.2021.116834 Xuwen Chen , Davide Vione , Thomas Borch , Jian Wang , Yanzheng Gao
|
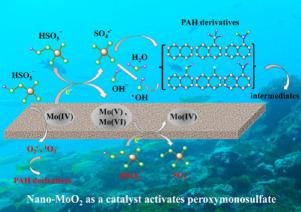
The rapid and efficient degradation of polycyclic aromatic hydrocarbon (PAH) derivatives with toxicological properties remains a substantial challenge. In this study, a cost-effective and eco-friendly catalyst, nano-MoO2 (0.05 g L−1), exhibited excellent performance in activating 4.0 mmol L−1 peroxymonosulfate (PMS) for the degradation of naphthalene derivatives with 1 mg L−1 in aqueous systems; these derivatives include 1-methylnaphthalene, 1-nitronaphthalene, 1-chloronaphthalene, 1-naphthylamine and 1-naphthol, with high degradation rates of 87.52%, 86.23%, 97.87%, 99.74%, and 77.16%. Nano-MoO2 acts as an electron donor by transferring an electron causing O-O bond of PMS to cleave producing SO4·−, and later ·OH. Electron paramagnetic resonance (EPR) analysis combined with free radical quenching research indicated that SO4·− and ·OH dominated the degradation of naphthalene derivatives, and O2·− and 1O2 participated in the processes. X-ray photoelectron spectroscopy (XPS) revealed the transformation of Mo(IV) to Mo(V) and Mo(VI), which suggested that the activation process proceeded via electron transfer from nano-MoO2 to PMS. The applicability of the nano-MoO2/PMS system in influencing parameters and stability was explored. The degradation pathways were primarily elucidated for each naphthalene derivative based on the intermediates identified in the systems. The -CH3, -NO2, -Cl, -OH substituents increased the positive electrostatic potential (ESP) on the molecular surface of 1-methylnaphthalene, 1-nitronaphthalene, 1-chloronaphthalene, and 1-naphthol, which reduced the electrophilic reaction and electron transfer between the reactive species and pollutants, leading to a lower degradation rate of naphthalene derivatives than the parent compound. However, the effect of -NH2 substituents is the opposite. These findings suggest that nano-MoO2 may aid as a novel catalyst in the future remediation of environments polluted with PAH derivatives.
中文翻译:

纳米MoO 2活化过氧单硫酸盐以降解PAH衍生物
具有毒理学性质的多环芳烃(PAH)衍生物的快速有效降解仍然是一个巨大的挑战。在这项研究中,一种经济高效且环保的催化剂,纳米MoO 2(0.05 g L -1),在活化4.0 mmol L -1过氧一硫酸盐(PMS)降解1 mg L萘衍生物方面表现出出色的性能。-1在水性体系中;这些衍生物包括1-甲基萘,1-硝基萘,1-氯萘,1-萘胺和1-萘酚,其高降解率分别为87.52%,86.23%,97.87%,99.74%和77.16%。纳米MoO 2通过转移电子使PMS的OO键裂解产生SO充当电子供体4 · −和更高版本· OH。电子顺磁共振(EPR)分析和自由基猝灭研究相结合表明,SO 4 · -和· OH主导了萘衍生物的降解,O 2 · -和1 O 2参与了该过程。X射线光电子能谱(XPS)揭示了Mo(IV)向Mo(V)和Mo(VI)的转化,这表明激活过程是通过电子从纳米MoO 2转移到PMS进行的。纳米MoO 2的适用性探索了/ PMS系统对参数和稳定性的影响。根据系统中鉴定的中间体,首先阐明了每种萘衍生物的降解途径。-CH 3,-NO 2,-Cl,-OH取代基增加了1-甲基萘,1-硝基萘,1-氯萘和1-萘酚的分子表面上的正静电势(ESP),从而降低了亲电反应以及反应性物质和污染物之间的电子转移,导致萘衍生物的降解率低于母体化合物。但是,-NH 2取代基的作用相反。这些发现表明,纳米MoO 2 可能在将来修复被PAH衍生物污染的环境方面作为一种新型催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号