Talanta ( IF 5.6 ) Pub Date : 2021-01-13 , DOI: 10.1016/j.talanta.2021.122096 Erik J. Oerter , Michael Singleton , Zurong Dai , Scott Donald , Melissa Thaw , M. Lee Davisson
|
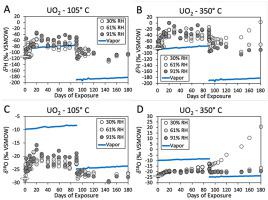
Hydrated secondary mineralization readily forms on the surface of UO2 particles exposed to humidity in an oxidizing environment. The oxygen stable isotope composition of the secondary uranium oxide may reflect that of the water vapor, as well as the hydrogen and oxygen stable isotopic composition of the mineral hydration water. The geospatial organization of δ2H and δ18O values of atmospheric humidity and precipitation is increasingly well understood, which suggests that the hydrogen and oxygen stable isotopes in secondary mineral hydration water may yield information on the environment in which the mineralization formed. UO2 powders were exposed to air with constant 30%, 61%, and 91% relative humidity, and constant H and O stable isotope composition. Aliquots were sampled from the UO2 materials at intervals of 1–10 days through the total humidity exposure duration of 180 days. Scanning electron microscopy, transmission electron microscopy, and x-ray diffraction analysis of the humidity-exposed UO2 indicates that schoepite/metaschoepite [(UO3)•2H2O] secondary phases had formed on the underlying UO2. The δ2H and δ18O values of mineral hydration waters were determined by thermogravimetry-enabled isotope ratio infrared spectroscopy (TGA-IRIS). Results indicate that hydrogen in the surface sorbed and mineral hydration waters is exchangeable and thus their δ2H values are difficult to interpret. However, oxygen in these waters is less exchangeable, and thus the oxygen stable isotope composition of the schoepite/metaschoepite hydration water is likely to be related to that of the exposure water vapor. After formation of schoepite/metaschoepite, the δ18O values of the hydration water in schoepite/metaschoepite does not change in response to changes in exposure vapor δ18O values, which suggests that the δ18O values of the hydration water is relatively durable. These findings suggest that information about the origin and storage history of a UO2 sample may be discernable from δ18O values of schoepite/metaschoepite hydration water.
中文翻译:

UO 2二次矿化中水化水的稳定同位素特征
在氧化环境中暴露于湿气的UO 2颗粒表面容易形成水合的二次矿化作用。次要铀氧化物的氧稳定同位素组成可以反映水蒸气的氧组成同位素,以及矿物水化水的氢和氧稳定同位素组成。的δ地理空间组织2 H和δ 18个大气湿度和降水的O值日益很好理解的,这表明氢气和氧气在稳定次级水合水同位素可以产生在其中形成的矿化的环境信息。UO 2粉末暴露在相对湿度恒定为30%,61%和91%,H和O稳定同位素组成恒定的空气中。从UO 2材料中抽出等分试样,时间间隔为1-10天,整个暴露时间为180天。暴露于湿气的UO 2的扫描电子显微镜,透射电子显微镜和X射线衍射分析表明,在下面的UO 2上已形成了钠钙辉石/变锰铁矿[(UO 3)•2H 2 O]二次相。的δ 2 H和δ 18矿物水合水的O值通过热重分析法同位素比红外光谱法(TGA-IRIS)确定。结果表明在表面的氢吸附和水合水是可更换的,因此他们的δ 2 ħ值是难以解释。但是,这些水中的氧难以交换,因此,钠铁矿/偏辉石水合水的氧稳定同位素组成可能与暴露水蒸气的氧稳定同位素组成有关。形成schoepite / metaschoepite中,δ的后18个在schoepite水合水的O值/ metaschoepite不响应于δ在曝光蒸气的变化而改变18个O值,这表明δ 18水合水的O值是相对耐用的。这些结果表明大约UO的起源和存储历史信息2样品可以是来自δ可辨别18个schoepite / metaschoepite水合水的O值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号