Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 5.2 ) Pub Date : 2021-01-13 , DOI: 10.1016/j.colsurfa.2021.126150 Yangyang Zhang , Jeremy B. Fein , Yilian Li , Qiang Yu , Bo Zu , Chunli Zheng
|
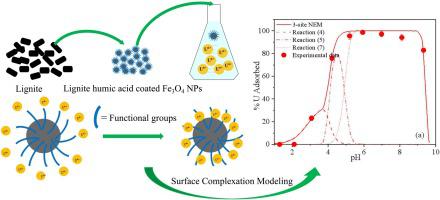
Lignite humic acid-coated Fe3O4 nanoparticles (LHA-coated Fe3O4 NPs) with different extents of LHA coating were synthesized and characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD), Fourier transformed infrared (FTIR), X-ray photoelectron spectroscopy (XPS) and potentiometric titration. The results of characterization revealed that LHA-coated Fe3O4 NPs contained plentiful oxygen-containing groups. Batch adsorption measurements show that U(VI) adsorption is pH dependent, with an optimum pH range of 5.0–8.0. The removal kinetics of U(VI) can be well fitted by the pseudo-second-order model. The maximum adsorption capacities at 298 K were 42.5, 55.6, and 68.7 mg g−1 on 0.5, 1.5, and 2.5 LHA-coated Fe3O4 NPs, respectively. The regeneration experiment showed that LHA-coated Fe3O4 NPs exhibited good recoverability for U(VI) adsorption. The adsorption mechanism of U(VI) on LHA-coated Fe3O4 NPs revealed by XPS and surface complexation model. XPS results indicate that both > Fe surface sites and oxygen-containing groups are involved in U(VI) adsorption. Moreover, no significant U(VI) reduction occurs on the surfaces of the LHA-coated Fe3O4 NPs, possibly because of the insulating properties of the LHA coating. Surface complexation modeling further demonstrated that U(VI) adsorption onto the Fe3O4 NPs with the highest extents of LHA coating is dominated by binding onto the LHA-associated binding sites (e.g., carboxyl sites), whereas > Fe surface sites are also involved in U(VI) adsorption onto Fe3O4 NPs with the lowest extent of LHA coating. LHA-coated Fe3O4 NPs represents a cost-efficient and good stability adsorbent for the effective removal of U(VI) from aqueous solutions, and our results provide the means for modeling the adsorption behavior as a function of the pH and extent of LHA coating.
中文翻译:

U(VI)对褐煤腐殖酸包覆的Fe 3 O 4纳米颗粒的吸附:实验测量和表面络合模型
合成了具有不同程度的LHA涂层的褐煤腐殖酸涂层的Fe 3 O 4纳米颗粒(LHA涂层的Fe 3 O 4 NPs),并通过扫描电子显微镜(SEM),X射线衍射(XRD),傅立叶变换红外光谱( FTIR),X射线光电子能谱(XPS)和电位滴定法。表征结果表明,LHA包覆的Fe 3 O 4NP含有大量的含氧基团。批量吸附测量表明,U(VI)吸附取决于pH,最佳pH范围为5.0–8.0。U(VI)的去除动力学可以通过拟二阶模型很好地拟合。298 K时在0.5、1.5和2.5 LHA涂覆的Fe 3 O 4 NPs上的最大吸附容量分别为42.5、55.6和68.7 mg g -1。再生实验表明,LHA包覆的Fe 3 O 4 NPs具有良好的U(VI)吸附回收率。U(VI)在LHA包覆的Fe 3 O 4上的吸附机理XPS和表面络合模型揭示了NP。XPS结果表明> Fe表面位点和含氧基团都参与U(VI)吸附。此外,可能由于LHA涂层的绝缘性,在LHA涂层的Fe 3 O 4 NPs的表面上不会发生明显的U(VI)还原。表面络合模型进一步证明,U(VI)吸附到LHA覆盖程度最高的Fe 3 O 4 NPs上主要是通过结合到LHA相关的结合位点(例如羧基位点)上,而> Fe表面位点也是涉及U(VI)吸附到LHA涂层程度最低的Fe 3 O 4 NP上。LHA涂层的铁3O 4 NPs是一种从水溶液中有效去除U(VI)的经济高效且稳定性好的吸附剂,我们的结果提供了根据pH和LHA涂层程度对吸附行为进行建模的方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号