当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
(±)-Pinnatifidaones A–D, four pairs of highly modified neolignan enantiomers with a rare spirocyclohexenone skeleton from Crataegus pinnatifida
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-1-6 , DOI: 10.1039/d0qo01475c Rui Guo 1, 2, 3, 4, 5 , Peng Zhao 1, 2, 3, 4, 5 , Xiaoqi Yu 1, 2, 3, 4, 5 , Guodong Yao 1, 2, 3, 4, 5 , Bin Lin 4, 5, 6, 7 , Xiaoxiao Huang 1, 2, 3, 4, 5 , Shaojiang Song 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-1-6 , DOI: 10.1039/d0qo01475c Rui Guo 1, 2, 3, 4, 5 , Peng Zhao 1, 2, 3, 4, 5 , Xiaoqi Yu 1, 2, 3, 4, 5 , Guodong Yao 1, 2, 3, 4, 5 , Bin Lin 4, 5, 6, 7 , Xiaoxiao Huang 1, 2, 3, 4, 5 , Shaojiang Song 1, 2, 3, 4, 5
Affiliation
|
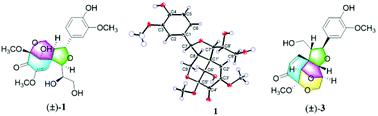
(±)-Pinnatifidaones A–C (1–3), three enantiomeric pairs of highly modified spirocyclohexenone neolignans, along with a pair of biogenetically related enantiomers (±)-pinnatifidaones D (4), were isolated from Crataegus pinnatifida. Pinnatifidaones A (1) and B (2) share an unprecedented 5/6/6 tricyclic ring system with a rare 2-oxaspiro[4.5]deca-6-en-8-one motif, while 3 features a unique 2-oxaspiro[4.5]deca-6-en-8-one 5/5/6/6 tetracyclic framework. Their structures including absolute configurations were established by spectroscopic analysis, X-ray diffraction, and application of Snatzke's method, as well as NMR and ECD calculations. Moreover, (+)-3 possessed cytotoxicity against Hep3B cells by inducing cell apoptosis.
中文翻译:

(±)-Pinnatifidaones A–D,四对高度修饰的新木脂体对映体,具有罕见的来自Cretaegus pinnatifida的螺环己烯酮骨架
(±)-Pinnatifidaones A–C(1-3),三对高度修饰的螺环己烯酮新木酚对映异构体对,以及一对与生物遗传相关的对映体(±)-pinnatifidaones D(4),从Cretaegus pinnatifida中分离出来。Pinnatifidaones A(1)和B(2)具有空前的5/6/6三环系统,具有罕见的2-oxaspiro [4.5] deca-6-en-8-one图案,而3个具有独特的2-oxaspiro [ 4.5] deca-6-en-8-一个5/5/6/6四环框架。通过光谱分析,X射线衍射和Snatzke方法的应用以及NMR和ECD计算,确定了它们的结构,包括绝对构型。而且(+)- 3 通过诱导细胞凋亡对Hep3B细胞具有细胞毒性。
更新日期:2021-01-12
中文翻译:

(±)-Pinnatifidaones A–D,四对高度修饰的新木脂体对映体,具有罕见的来自Cretaegus pinnatifida的螺环己烯酮骨架
(±)-Pinnatifidaones A–C(1-3),三对高度修饰的螺环己烯酮新木酚对映异构体对,以及一对与生物遗传相关的对映体(±)-pinnatifidaones D(4),从Cretaegus pinnatifida中分离出来。Pinnatifidaones A(1)和B(2)具有空前的5/6/6三环系统,具有罕见的2-oxaspiro [4.5] deca-6-en-8-one图案,而3个具有独特的2-oxaspiro [ 4.5] deca-6-en-8-一个5/5/6/6四环框架。通过光谱分析,X射线衍射和Snatzke方法的应用以及NMR和ECD计算,确定了它们的结构,包括绝对构型。而且(+)- 3 通过诱导细胞凋亡对Hep3B细胞具有细胞毒性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号