当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aromatic nucleophilic substitution (snar) reactions of halo‐substituted dinitrobenzene in liposome reaction media: Effect of reaction medium and role of halogen leaving group
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2021-01-11 , DOI: 10.1002/poc.4182 Jyoti Dutta 1 , Shraeddha Tiwari 1
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2021-01-11 , DOI: 10.1002/poc.4182 Jyoti Dutta 1 , Shraeddha Tiwari 1
Affiliation
|
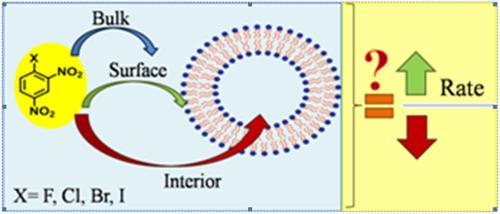
SNAr reactions constitute an important pathway for the synthesis of many crucial organic derivatives from polyhaloaromatic compounds. The sluggish nature of the reaction in many cases makes it a challenging pathway and limits its potential applications. In the present report, liposomes have been used as model membrane systems to study nucleophilic substitution reactions of halo‐substituted dinitrobenzene with morpholine. The results show an interesting dependence of the reactivity on the size and composition of liposomes. The extent of rate acceleration in liposomes is strongly dependent on the identity of the halogen which undergoes the substitution—for example, chloro‐substituted aromatic compounds show the most sensitivity to the presence of lipsomal reaction media. The observed behavior correlates with the reported reactivity of halobenzenes while revealing interesting details which may be critical in harnessing the reactivity of less reactive substrates. The results explore the viability of employing liposomes as promising alternatives for synthetic protocols.
中文翻译:

脂质体反应介质中卤代二硝基苯的芳香亲核取代(snar)反应:反应介质的影响和卤素离去基团的作用
小号ñAr反应构成了从多卤代芳香族化合物合成许多关键有机衍生物的重要途径。在许多情况下,反应的缓慢性质使其成为具有挑战性的途径,并限制了其潜在的应用。在本报告中,脂质体已用作模型膜系统,以研究卤代二硝基苯与吗啉的亲核取代反应。结果显示,反应性对脂质体的大小和组成具有令人感兴趣的依赖性。脂质体中速率加速的程度在很大程度上取决于进行取代的卤素的身份-例如,氯取代的芳族化合物对脂质体反应介质的存在最敏感。观察到的行为与所报道的卤代苯的反应性相关,同时揭示了有趣的细节,这对于利用反应性较低的底物的反应性可能至关重要。结果探索了将脂质体用作合成方案的有前途的替代方法的可行性。
更新日期:2021-01-11
中文翻译:

脂质体反应介质中卤代二硝基苯的芳香亲核取代(snar)反应:反应介质的影响和卤素离去基团的作用
小号ñAr反应构成了从多卤代芳香族化合物合成许多关键有机衍生物的重要途径。在许多情况下,反应的缓慢性质使其成为具有挑战性的途径,并限制了其潜在的应用。在本报告中,脂质体已用作模型膜系统,以研究卤代二硝基苯与吗啉的亲核取代反应。结果显示,反应性对脂质体的大小和组成具有令人感兴趣的依赖性。脂质体中速率加速的程度在很大程度上取决于进行取代的卤素的身份-例如,氯取代的芳族化合物对脂质体反应介质的存在最敏感。观察到的行为与所报道的卤代苯的反应性相关,同时揭示了有趣的细节,这对于利用反应性较低的底物的反应性可能至关重要。结果探索了将脂质体用作合成方案的有前途的替代方法的可行性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号