当前位置:
X-MOL 学术
›
Cell Prolif.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NLRX1/FUNDC1/NIPSNAP1‐2 axis regulates mitophagy and alleviates intestinal ischaemia/reperfusion injury
Cell Proliferation ( IF 5.9 ) Pub Date : 2021-01-11 , DOI: 10.1111/cpr.12986 Shaoqin Li 1 , Yi Zhou 1 , Xiaocheng Gu 1 , Xiaoping Zhang 2, 3 , Zhongzhi Jia 1
Cell Proliferation ( IF 5.9 ) Pub Date : 2021-01-11 , DOI: 10.1111/cpr.12986 Shaoqin Li 1 , Yi Zhou 1 , Xiaocheng Gu 1 , Xiaoping Zhang 2, 3 , Zhongzhi Jia 1
Affiliation
|
OBJECTIVES
Mitophagy is considered to be a key mechanism in the pathogenesis of intestinal ischaemic reperfusion (IR) injury. NOD-like receptor X1 (NLRX1) is located in the mitochondria and is highly expressed in the intestine, and is known to modulate ROS production, mitochondrial damage, autophagy and apoptosis. However, the function of NLRX1 in intestinal IR injury is unclear. MATERIALS AND METHODS
NLRX1 in rats with IR injury or in IEC-6 cells with hypoxia reoxygenation (HR) injury were measured by Western blotting, real-time PCR and immunohistochemistry. The function of NLRX1-FUNDC1-NIPSNAP1/NIPSNAP2 axis in mitochondrial homeostasis and cell apoptosis were assessed in vitro. RESULTS
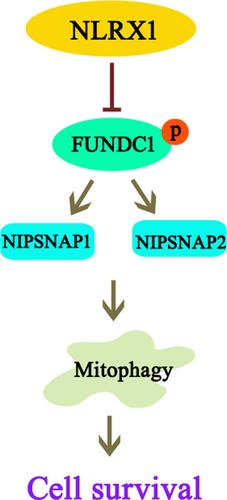
NLRX1 is significantly downregulated following intestinal IR injury. In vivo studies showed that rats overexpressing NLRX1 exhibited resistance against intestinal IR injury and mitochondrial dysfunction. These beneficial effects of NLRX1 overexpression were dependent on mitophagy activation. Functional studies showed that HR injury reduced NLRX1 expression, which promoted phosphorylation of FUN14 domain-containing 1 (FUNDC1). Based on immunoprecipitation studies, it was evident that phosphorylated FUNDC1 could not interact with the mitophagy signalling proteins NIPSNAP1 and NIPSNAP2 on the outer membrane of damaged mitochondria, which failed to launch the mitophagy process, resulting in the accumulation of damaged mitochondria and epithelial apoptosis. CONCLUSIONS
NLRX1 regulates mitophagy via FUNDC1-NIPSNAP1/NIPSNAP2 signalling pathway. Thus, this study provides a potential target for the development of a therapeutic strategy for intestinal IR injury.
中文翻译:

NLRX1/FUNDC1/NIPSNAP1-2轴调节线粒体自噬并减轻肠道缺血/再灌注损伤
目的 线粒体自噬被认为是肠道缺血再灌注(IR)损伤发病机制的关键机制。 NOD 样受体 X1 (NLRX1) 位于线粒体中,在肠道中高表达,已知可调节 ROS 产生、线粒体损伤、自噬和细胞凋亡。然而,NLRX1 在肠道 IR 损伤中的功能尚不清楚。材料和方法 通过蛋白质印迹、实时 PCR 和免疫组织化学测定 IR 损伤大鼠或缺氧复氧 (HR) 损伤的 IEC-6 细胞中的 NLRX1。体外评估NLRX1-FUNDC1-NIPSNAP1/NIPSNAP2轴在线粒体稳态和细胞凋亡中的功能。结果 NLRX1 在肠道 IR 损伤后显着下调。体内研究表明,过度表达 NLRX1 的大鼠表现出对肠道 IR 损伤和线粒体功能障碍的抵抗力。 NLRX1 过表达的这些有益作用依赖于线粒体自噬激活。功能研究表明,HR 损伤降低了 NLRX1 的表达,从而促进 FUN14 结构域包含 1 (FUNDC1) 的磷酸化。基于免疫沉淀研究,磷酸化的FUNDC1不能与受损线粒体外膜上的线粒体自噬信号蛋白NIPSNAP1和NIPSNAP2相互作用,从而无法启动线粒体自噬过程,导致受损线粒体的积累和上皮细胞凋亡。结论 NLRX1 通过 FUNDC1-NIPSNAP1/NIPSNAP2 信号通路调节线粒体自噬。因此,这项研究为开发肠道IR损伤治疗策略提供了一个潜在的目标。
更新日期:2021-01-11
中文翻译:

NLRX1/FUNDC1/NIPSNAP1-2轴调节线粒体自噬并减轻肠道缺血/再灌注损伤
目的 线粒体自噬被认为是肠道缺血再灌注(IR)损伤发病机制的关键机制。 NOD 样受体 X1 (NLRX1) 位于线粒体中,在肠道中高表达,已知可调节 ROS 产生、线粒体损伤、自噬和细胞凋亡。然而,NLRX1 在肠道 IR 损伤中的功能尚不清楚。材料和方法 通过蛋白质印迹、实时 PCR 和免疫组织化学测定 IR 损伤大鼠或缺氧复氧 (HR) 损伤的 IEC-6 细胞中的 NLRX1。体外评估NLRX1-FUNDC1-NIPSNAP1/NIPSNAP2轴在线粒体稳态和细胞凋亡中的功能。结果 NLRX1 在肠道 IR 损伤后显着下调。体内研究表明,过度表达 NLRX1 的大鼠表现出对肠道 IR 损伤和线粒体功能障碍的抵抗力。 NLRX1 过表达的这些有益作用依赖于线粒体自噬激活。功能研究表明,HR 损伤降低了 NLRX1 的表达,从而促进 FUN14 结构域包含 1 (FUNDC1) 的磷酸化。基于免疫沉淀研究,磷酸化的FUNDC1不能与受损线粒体外膜上的线粒体自噬信号蛋白NIPSNAP1和NIPSNAP2相互作用,从而无法启动线粒体自噬过程,导致受损线粒体的积累和上皮细胞凋亡。结论 NLRX1 通过 FUNDC1-NIPSNAP1/NIPSNAP2 信号通路调节线粒体自噬。因此,这项研究为开发肠道IR损伤治疗策略提供了一个潜在的目标。











































 京公网安备 11010802027423号
京公网安备 11010802027423号