Journal of Environmental Chemical Engineering ( IF 7.7 ) Pub Date : 2021-01-12 , DOI: 10.1016/j.jece.2021.105062 Ana Paula Nazar de Souza , Yordy E. Licea , Marcos V. Colaço , Jaqueline D. Senra , Nakédia M.F. Carvalho
|
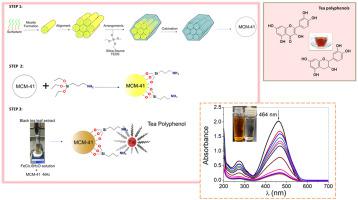
In this work, green iron oxide nanoparticles (FeONPs) were prepared from Fe(III) precursor and Camellia sinensis (black tea) extract, and were anchored on amino-functionalized mesoporous silica MCM-41. The multicomponent composite named BTFe/MCM-41-NH2 was characterized and applied in the adsorption of the anionic azo dye methyl orange. The results indicated that BTFe/MCM-41-NH2 is 11 times more adsorbent than MCM-41 and 2.5 times more adsorbent than MCM-41-NH2, showing the improved adsorption property of the multifunctional material. Adsorption parameters such as adsorbent dosage, initial dye concentration, pH and time, were screened for BTFe/MCM-41-NH2, a provided an experimental adsorption capacity at equilibrium of qe,exp = 105.3 mg g−1 at 298 K. Zeta potential measurements showed the favored electrostatic interaction with the negatively charged dye at acid pH when BTFe/MCM-41-NH2 is positively charged. Kinetic studies revealed pseudo-first-order model characteristic of reaction-controlled adsorption. The experimental data at equilibrium was fit by several adsorption isotherms models. Freundlich and Temkin were the most suitable isotherms to describe the prepared material, suggesting an adsorption through a heterogeneous surface with multiple sites of adsorption, such as protonated amino groups, FeONPs, besides the tea polyphenols that provided extra sites of adsorption through π-π interactions with the aromatic rings of the dyes.
中文翻译:

绿色氧化铁/氨基官能化的MCM-41复合材料作为阴离子偶氮染料的吸附剂:动力学和等温线研究
在这项工作中,从Fe(III)前体和山茶(红茶)提取物制备了绿色氧化铁纳米颗粒(FeONPs),并将其锚定在氨基官能化的介孔二氧化硅MCM-41上。表征了多组分复合物BT Fe / MCM-41-NH 2,并将其应用于阴离子偶氮染料甲基橙的吸附。结果表明,BT的Fe / MCM-41-NH 2比MCM-41和比MCM-41-NH 2.5倍以上的吸附11倍的吸附剂更2,示出了多官能材料的改进的吸附性能。筛选了BT Fe / MCM-41-NH的吸附参数,如吸附剂量,初始染料浓度,pH和时间参照图2,a提供了在298 K下平衡为q e,exp = 105.3 mg g -1时的实验吸附容量。Zeta电势测量显示在BT Fe / MCM-41-NH的酸性pH下,与带负电荷的染料发生有利的静电相互作用2带正电。动力学研究揭示了反应控制吸附的拟一阶模型特征。几个吸附等温线模型拟合了平衡时的实验数据。Freundlich和Temkin是最合适的等温线,用于描述所制备的材料,这表明它通过具有多个吸附位点(如质子化的氨基,FeONPs)的异质表面进行吸附,此外茶多酚通过π-π相互作用提供了额外的吸附位点。与染料的芳香环。



























 京公网安备 11010802027423号
京公网安备 11010802027423号