Molecular Catalysis ( IF 3.9 ) Pub Date : 2021-01-12 , DOI: 10.1016/j.mcat.2021.111394 Liying Mu , Wenjing Fan , Xiang-Ai Yuan , Congcong Huang , Dan Li , Siwei Bi
|
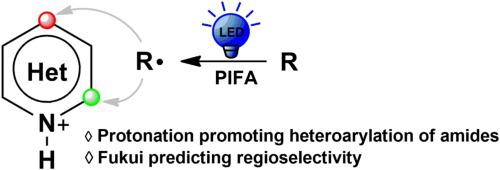
A computational study is carried out to understand the mechanism and excellent regioselectivity in metal-free heteroarylation of amides reported by Zhu’s group. The heteroarylation reaction started with the initial generation of key nitrogen-centered radicals via ligand exchange between reactant 1a and initiator PIFA under visible-light irradiation. Following, this reaction undergoes four-stages: 1,5-hydrogen atom transfer, C C coupling, single electron transfer and proton transfer. The C
C coupling, single electron transfer and proton transfer. The C C coupling step is identified as the selectivity-determining step in which the carbon-centered radical (C) selectively only attacks the carbon atom adjacent to nitrogen of lepidine (2a). And the radical C more easily attacks the protonated 2a, compared with unprotonated 2a, due to significantly lowered SOMO/LUMO energy difference between them to promote this nucleophilic radical addition. From the calculated result, we can see that the positive effect of the acidity of the reaction substrates on the nucleophilic addition to heteroarenes. Fukui functions of different types of heteroarene substrates are calculated to predict the favorable nucleophilic sites. The calculated most favorable reactive sites of heteroarene substrates are well consistent with the experimental observed ones. This theoretical research provides deeper understandings for the underlying mechanism and the origin of exclusive regioselectivity of the heteroarylation of amides.
C coupling step is identified as the selectivity-determining step in which the carbon-centered radical (C) selectively only attacks the carbon atom adjacent to nitrogen of lepidine (2a). And the radical C more easily attacks the protonated 2a, compared with unprotonated 2a, due to significantly lowered SOMO/LUMO energy difference between them to promote this nucleophilic radical addition. From the calculated result, we can see that the positive effect of the acidity of the reaction substrates on the nucleophilic addition to heteroarenes. Fukui functions of different types of heteroarene substrates are calculated to predict the favorable nucleophilic sites. The calculated most favorable reactive sites of heteroarene substrates are well consistent with the experimental observed ones. This theoretical research provides deeper understandings for the underlying mechanism and the origin of exclusive regioselectivity of the heteroarylation of amides.
中文翻译:

酰胺的C(sp 3)-H杂芳基化的机理见解和区域选择性的Fukui功能分析
进行了计算研究,以了解朱氏研究小组报道的酰胺的无金属杂芳基化反应的机理和优异的区域选择性。杂芳基化反应开始于在可见光照射下,通过反应物1a和引发剂PIFA之间的配体交换,最初生成关键的氮中心自由基。随后,该反应经历四个阶段:1,5-氢原子转移, CC偶联,单电子转移和质子转移。C
CC偶联,单电子转移和质子转移。C  C偶联步骤被认为是选择性确定步骤,其中以碳为中心的基团(C)仅选择性地攻击与哌啶(2a)的氮相邻的碳原子。和部首C更容易地攻击质子化2a中,与未质子化的比较图2a,由于它们之间的显著降低SOMO / LUMO的能量差,以促进此亲核基加。从计算结果,我们可以看到反应底物的酸度对杂芳烃亲核加成的积极影响。计算不同类型的杂芳烃底物的Fukui功能以预测有利的亲核位点。计算出的杂芳烃底物最有利的反应中心与实验观察到的一致。这项理论研究为酰胺杂芳基化的基本机理和排他性区域选择性的起源提供了更深刻的理解。
C偶联步骤被认为是选择性确定步骤,其中以碳为中心的基团(C)仅选择性地攻击与哌啶(2a)的氮相邻的碳原子。和部首C更容易地攻击质子化2a中,与未质子化的比较图2a,由于它们之间的显著降低SOMO / LUMO的能量差,以促进此亲核基加。从计算结果,我们可以看到反应底物的酸度对杂芳烃亲核加成的积极影响。计算不同类型的杂芳烃底物的Fukui功能以预测有利的亲核位点。计算出的杂芳烃底物最有利的反应中心与实验观察到的一致。这项理论研究为酰胺杂芳基化的基本机理和排他性区域选择性的起源提供了更深刻的理解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号