Gene ( IF 2.6 ) Pub Date : 2021-01-12 , DOI: 10.1016/j.gene.2021.145427 Veronika Pražienková , Jiří Funda , Zdenko Pirník , Alena Karnošová , Lucie Hrubá , Lucia Kořínková , Barbora Neprašová , Petra Janovská , Michal Benzce , Michaela Kadlecová , Jaroslav Blahoš , Jan Kopecký , Blanka Železná , Jaroslav Kuneš , Kristina Bardová , Lenka Maletínská
|
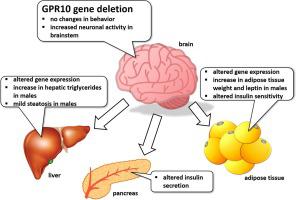
G-protein-coupled receptor GPR10 is expressed in brain areas regulating energy metabolism. In this study, the effects of GPR10 gene deficiency on energy homeostasis in mice of both sexes fed either standard chow or a high-fat diet (HFD) were studied, with a focus on neuronal activation of PrRP neurons, and adipose tissue and liver metabolism. GPR10 deficiency in males upregulated the phasic and tonic activity of PrRP neurons in the nucleus of the solitary tract. GPR10 knockout (KO) males on a standard diet displayed a higher body weight than their wild-type (WT) littermates due to an increase in adipose tissue mass; however, HFD feeding did not cause weight differences between genotypes. Expression of lipogenesis genes was suppressed in the subcutaneous adipose tissue of GPR10 KO males. In contrast, GPR10 KO females did not differ in body weight from their WT controls, but showed elevated expression of lipid metabolism genes in the liver and subcutaneous adipose tissue compared to WT controls. An attenuated non-esterified fatty acids change after glucose load compared to WT controls suggested a defect in insulin-mediated suppression of lipolysis in GPR10 KO females. Indirect calorimetry did not reveal any differences in energy expenditure among groups. In conclusion, deletion of GPR10 gene resulted in changes in lipid metabolism in mice of both sexes, however in different extent. An increase in adipose tissue mass observed in only GPR10 KO males may have been prevented in GPR10 KO females owing to a compensatory increase in the expression of metabolic genes.
中文翻译:

小鼠GPR10基因缺失增加基础神经元活性,干扰胰岛素敏感性并改变脂质稳态
G蛋白偶联受体GPR10在调节能量代谢的大脑区域表达。在这项研究中,研究了GPR10基因缺陷对标准饮食或高脂饮食(HFD)喂养的两性小鼠的能量稳态的影响,重点是PrRP神经元的神经元活化,脂肪组织和肝脏代谢。男性中的GPR10缺乏上调了孤立道核中PrRP神经元的相位和滋补活性。由于脂肪组织质量的增加,采用标准饮食的GPR10基因敲除(KO)雄性的体重比其野生型(WT)同窝的雄性高。但是,HFD饲喂不会引起基因型之间的体重差异。脂肪生成基因的表达在GPR10 KO男性的皮下脂肪组织中受到抑制。相反,GPR10 KO雌性与野生型对照体重无差异,但与野生型对照相比,肝脏和皮下脂肪组织中脂质代谢基因的表达升高。与WT对照相比,葡萄糖负荷后非酯化脂肪酸的衰减减弱,提示GPR10 KO女性的胰岛素介导的脂解抑制作用存在缺陷。间接量热法未显示组间能量消耗的任何差异。总之,GPR10基因的缺失导致雌性和雌性小鼠脂质代谢的改变,但是程度不同。由于代谢基因表达的补偿性增加,在GPR10 KO雌性中仅可预防仅在GPR10 KO雄性中观察到的脂肪组织量增加。但与WT对照相比,在肝脏和皮下脂肪组织中脂质代谢基因的表达升高。与WT对照相比,葡萄糖负荷后非酯化脂肪酸的衰减减弱,提示GPR10 KO女性的胰岛素介导的脂解抑制作用存在缺陷。间接量热法未显示组间能量消耗的任何差异。总之,GPR10基因的缺失导致雌性和雌性小鼠脂质代谢的改变,但是程度不同。由于代谢基因表达的补偿性增加,在GPR10 KO雌性中仅可预防仅在GPR10 KO雄性中观察到的脂肪组织量增加。但与WT对照相比,在肝脏和皮下脂肪组织中脂质代谢基因的表达升高。与WT对照相比,葡萄糖负荷后非酯化脂肪酸的衰减减弱,提示GPR10 KO女性的胰岛素介导的脂解抑制作用存在缺陷。间接量热法未显示组间能量消耗的任何差异。总之,GPR10基因的缺失导致雌性和雌性小鼠脂质代谢的改变,但是程度不同。由于代谢基因表达的补偿性增加,在GPR10 KO雌性中仅可预防仅在GPR10 KO雄性中观察到的脂肪组织量增加。与WT对照相比,葡萄糖负荷后非酯化脂肪酸的衰减减弱,提示GPR10 KO女性的胰岛素介导的脂解抑制作用存在缺陷。间接量热法未显示组间能量消耗的任何差异。总之,GPR10基因的缺失导致雌性和雌性小鼠脂质代谢的改变,但是程度不同。由于代谢基因表达的补偿性增加,在GPR10 KO雌性中仅可预防仅在GPR10 KO雄性中观察到的脂肪组织量增加。与WT对照相比,葡萄糖负荷后非酯化脂肪酸的衰减减弱,提示GPR10 KO女性的胰岛素介导的脂解抑制作用存在缺陷。间接量热法未显示组间能量消耗的任何差异。总之,GPR10基因的缺失导致雌性和雌性小鼠脂质代谢的改变,但是程度不同。由于代谢基因表达的补偿性增加,在GPR10 KO雌性中仅可预防仅在GPR10 KO雄性中观察到的脂肪组织量增加。但是程度不同。由于代谢基因表达的补偿性增加,在GPR10 KO雌性中仅可预防仅在GPR10 KO雄性中观察到的脂肪组织量增加。但是程度不同。由于代谢基因表达的补偿性增加,在GPR10 KO雌性中仅可预防仅在GPR10 KO雄性中观察到的脂肪组织量增加。











































 京公网安备 11010802027423号
京公网安备 11010802027423号