Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2021-01-12 , DOI: 10.1016/j.bioorg.2021.104654 Güler Yagiz 1 , Samir Abbas Ali Noma 2 , Aliye Altundas 1 , Khattab Al-Khafaji 3 , Tugba Taskin-Tok 4 , Burhan Ates 2
|
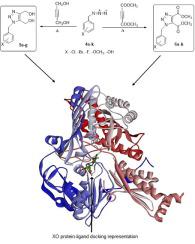
This study focused on synthesis various dimethyl N-benzyl-1H-1,2,3-triazole-4,5-dicarboxylate and (N-benzyl-1H-1,2,3-triazole-4,5-diyl)dimethanol derivatives under the conditions of green chemistry without the use of solvent and catalysts. Their inhibition properties were also investigated on xanthine oxidase (XO) activity. All dimethanol and dicarboxylate derivatives exhibited significant inhibition activities with IC50 values ranging from 0.71 to 2.25 μM. Especially, (1-(3-bromobenzyl)-1H-1,2,3-triazole-4,5-diyl)dimethanol (5c) and dimethyl 1-(4-chlorobenzyl)-1H-1,2,3-triazole-4,5-dicarboxylate (6 g) compounds were found to be the most promising derivatives on the XO enzyme inhibition with IC50 values 0.71 and 0.73 μM, respectively. Moreover, the double docking procedure was to evaluate compound modes of inhibition and their interactions with the protein (XO) at atomic level. Surprisingly, the docking results showed a good correlation with IC50 [correlation coefficient (R2 = 0.7455)]. Also, the docking results exhibited that the 5c, 6f and 6 g have lowest docking scores −4.790, −4.755, and −4.730, respectively. These data were in agreement with the IC50 values. These results give promising beginning stages to assist in the improvement of novel and powerful inhibitor against XO.
中文翻译:

N-benzyl-1H-1,2,3-triazole-4,5-dicarboxylate和(N-benzyl-1H-1,2,3-triazole-4, 5-二基)二甲醇衍生物
本研究重点合成了各种N - benzyl- 1H -1,2,3 -triazole-4,5-dicarboxylate和( N - benzyl- 1H -1,2,3 -triazole-4,5-diyl)在不使用溶剂和催化剂的情况下,在绿色化学条件下制备二甲醇衍生物。还研究了它们对黄嘌呤氧化酶 (XO) 活性的抑制特性。所有二甲醇和二羧酸衍生物均表现出显着的抑制活性,IC 50值范围为 0.71 至 2.25 μM。特别是,(1-(3-溴苄基)-1 ħ 1,2,3-三唑-4,5-二基)二甲醇(5C)和二甲基1-(4-氯苄基)-1 ħ 1,2,3- -triazole-4,5-dicarboxylate ( 6 g) 化合物被发现是最有希望的 XO 酶抑制衍生物,IC 50值分别为 0.71 和 0.73 μM。此外,双对接程序是在原子水平上评估复合抑制模式及其与蛋白质 (XO) 的相互作用。令人惊讶的是,对接结果显示出与 IC 50 [相关系数 (R 2 = 0.7455)]的良好相关性。此外,对接结果表明,5c、6f和6g 分别具有最低的对接分数 -4.790、-4.755 和 -4.730。这些数据与 IC 50 一致值。这些结果为帮助改进新型强效 XO 抑制剂提供了有希望的开始阶段。
































 京公网安备 11010802027423号
京公网安备 11010802027423号