Archives of Biochemistry and Biophysics ( IF 3.8 ) Pub Date : 2021-01-12 , DOI: 10.1016/j.abb.2020.108748 Wilson A. Tárraga , Lisandro J. Falomir-Lockhart , Horacio A. Garda , Marina C. González
|
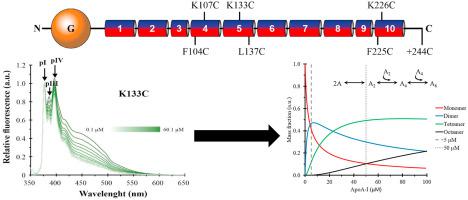
ApoA-I is the main protein of HDL which has anti-atherogenic properties attributed to reverse cholesterol transport. It shares with other exchangeable apolipoproteins a high level of structural plasticity. In the lipid-free state, the apolipoprotein amphipathic α-helices interact intra- and inter-molecularly, providing structural stabilization by a complex self-association mechanism. In this study, we employed a multi-parametric fluorescent probe to study the self-association of apoA-I. We constructed six single cysteine mutants spanning positions along three helices: F104C, K107C (H4), K133C, L137C (H5), F225C and K226C (H10); and labelled them with N-Maleimide Pyrene. Taking advantage of its spectral properties, namely formation of an excited dimer (excimer) and polarity-dependent changes in its fluorescence fine structure (P-value), we monitored the apoA-I self-association in its lipid-free form as a function of its concentration. Interactions in helices H5 (K133C) and H10 (F225C and K226C) were highlighted by excimer emission; while polarity changes were reported in helix H4 (K107C), as well as in helices H5 and H10. Mathematical models were developed to enrich data analysis and estimate association constants (KA) and oligomeric species distribution. Furthermore, we briefly discuss the usefulness of the multi-parametric fluorescent probe to monitor different equilibria, even at a single labelling position. Results suggest that apoA-I self-association must be considered to fully understand its physiological roles. Particularly, some contacts that stabilize discoidal HDL particles seem to be already present in the lipid-free apoA-I oligomers.
中文翻译:

solution标记载脂蛋白AI寡聚在溶液中的分析:光谱解卷积以及P值和准分子形成的变化
ApoA-I是HDL的主要蛋白,具有抗动脉粥样硬化特性,可归因于胆固醇逆向转运。它与其他可交换载脂蛋白共享高水平的结构可塑性。在无脂状态下,载脂蛋白两亲性α-螺旋分子内和分子间相互作用,通过复杂的自缔合机制提供结构稳定作用。在这项研究中,我们采用了多参数荧光探针来研究apoA-I的自缔合。我们构建了六个单半胱氨酸突变体,它们沿三个螺旋分布:F104C,K107C(H4),K133C,L137C(H5),F225C和K226C(H10);并用N-马来酰亚胺P标记。利用其光谱特性,即形成激发的二聚体(准分子)和其荧光精细结构的极性相关变化(P值),我们以无脂形式监测apoA-I自缔合与其浓度的关系。准分子发射突出了H5(K133C)和H10(F225C和K226C)螺旋中的相互作用。而在螺旋H4(K107C)以及螺旋H5和H10中报告了极性变化。开发了数学模型以丰富数据分析并估计关联常数(K A)和低聚物种分布。此外,我们简要讨论了即使在单个标记位置,多参数荧光探针也可用于监视不同的平衡。结果表明,必须考虑apoA-I自缔合才能充分了解其生理作用。特别地,稳定盘状HDL颗粒的一些接触似乎已经存在于无脂质的apoA-I低聚物中。









































 京公网安备 11010802027423号
京公网安备 11010802027423号