当前位置:
X-MOL 学术
›
RSC Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Substrate inhibition by the blockage of product release and its control by tunnel engineering
RSC Chemical Biology ( IF 4.2 ) Pub Date : 2021-1-11 , DOI: 10.1039/d0cb00171f Piia Kokkonen 1 , Andy Beier 1, 2 , Stanislav Mazurenko 1 , Jiri Damborsky 1, 2 , David Bednar 1, 2 , Zbynek Prokop 1, 2
RSC Chemical Biology ( IF 4.2 ) Pub Date : 2021-1-11 , DOI: 10.1039/d0cb00171f Piia Kokkonen 1 , Andy Beier 1, 2 , Stanislav Mazurenko 1 , Jiri Damborsky 1, 2 , David Bednar 1, 2 , Zbynek Prokop 1, 2
Affiliation
|
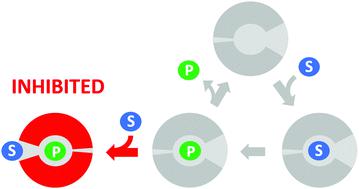
Substrate inhibition is the most common deviation from Michaelis–Menten kinetics, occurring in approximately 25% of known enzymes. It is generally attributed to the formation of an unproductive enzyme–substrate complex after the simultaneous binding of two or more substrate molecules to the active site. Here, we show that a single point mutation (L177W) in the haloalkane dehalogenase LinB causes strong substrate inhibition. Surprisingly, a global kinetic analysis suggested that this inhibition is caused by binding of the substrate to the enzyme–product complex. Molecular dynamics simulations clarified the details of this unusual mechanism of substrate inhibition: Markov state models indicated that the substrate prevents the exit of the halide product by direct blockage and/or restricting conformational flexibility. The contributions of three residues forming the possible substrate inhibition site (W140A, F143L and I211L) to the observed inhibition were studied by mutagenesis. An unusual synergy giving rise to high catalytic efficiency and reduced substrate inhibition was observed between residues L177W and I211L, which are located in different access tunnels of the protein. These results show that substrate inhibition can be caused by substrate binding to the enzyme–product complex and can be controlled rationally by targeted amino acid substitutions in enzyme access tunnels.
中文翻译:

阻断产物释放对底物的抑制及其隧道工程的控制
底物抑制是米氏动力学最常见的偏差,大约 25% 的已知酶会发生这种情况。它通常归因于两个或多个底物分子同时结合到活性位点后形成非生产性酶-底物复合物。在这里,我们发现卤代烷脱卤酶 LinB 中的单点突变 (L177W) 会导致强烈的底物抑制。令人惊讶的是,整体动力学分析表明这种抑制是由底物与酶-产物复合物的结合引起的。分子动力学模拟阐明了这种不寻常的底物抑制机制的细节:马尔可夫态模型表明,底物通过直接阻断和/或限制构象灵活性来阻止卤化物产物的排出。通过诱变研究了形成可能的底物抑制位点的三个残基(W140A、F143L 和 I211L)对观察到的抑制的贡献。在位于蛋白质不同通道的残基 L177W 和 I211L 之间观察到了不寻常的协同作用,导致高催化效率和降低的底物抑制。这些结果表明,底物抑制可能是由底物与酶-产物复合物结合引起的,并且可以通过酶通道中的目标氨基酸取代来合理控制。
更新日期:2021-01-11
中文翻译:

阻断产物释放对底物的抑制及其隧道工程的控制
底物抑制是米氏动力学最常见的偏差,大约 25% 的已知酶会发生这种情况。它通常归因于两个或多个底物分子同时结合到活性位点后形成非生产性酶-底物复合物。在这里,我们发现卤代烷脱卤酶 LinB 中的单点突变 (L177W) 会导致强烈的底物抑制。令人惊讶的是,整体动力学分析表明这种抑制是由底物与酶-产物复合物的结合引起的。分子动力学模拟阐明了这种不寻常的底物抑制机制的细节:马尔可夫态模型表明,底物通过直接阻断和/或限制构象灵活性来阻止卤化物产物的排出。通过诱变研究了形成可能的底物抑制位点的三个残基(W140A、F143L 和 I211L)对观察到的抑制的贡献。在位于蛋白质不同通道的残基 L177W 和 I211L 之间观察到了不寻常的协同作用,导致高催化效率和降低的底物抑制。这些结果表明,底物抑制可能是由底物与酶-产物复合物结合引起的,并且可以通过酶通道中的目标氨基酸取代来合理控制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号