当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Can the relative positions (cis–trans) of ligands really modulate the coordination of NO in ruthenium nitrosyl complexes?
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2020-12-21 , DOI: 10.1039/d0nj05262k Renato Pereira Orenha 1, 2, 3, 4 , Graziele Cappato Guerra Silva 1, 2, 3, 4 , Nelson Henrique Morgon 4, 5, 6, 7 , Giovanni Finoto Caramori 4, 8, 9, 10, 11 , Renato Luis Tame Parreira 1, 2, 3, 4
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2020-12-21 , DOI: 10.1039/d0nj05262k Renato Pereira Orenha 1, 2, 3, 4 , Graziele Cappato Guerra Silva 1, 2, 3, 4 , Nelson Henrique Morgon 4, 5, 6, 7 , Giovanni Finoto Caramori 4, 8, 9, 10, 11 , Renato Luis Tame Parreira 1, 2, 3, 4
Affiliation
|
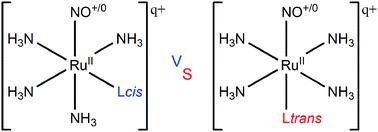
Nitric oxide is involved in a series of biological processes. Ruthenium tetraammine complexes are model structures to control the NO availability. The influence of ligands is critical to determine the Ru–NO bond stability. Herein, the ligands of different natures and charges, namely, σ-donors (NH3 and H−), (ii) π-donors (H2O and NH2−), and (iii) σ-donors/π-acceptors (CO and CN−) were evaluated relative to the effect promoted by same ligands not only in the cis position to the NO group, but also in the trans position to NO. The energy decomposition analysis shows linear Ru–NO+ and bent Ru–NO0 bonds in ruthenium tetraammine complexes independent of ligands in cis or trans positions with regard to the NO group. Overall, the substitution of one σ-donor ligand with one π-donor ligand in the cis position to the NO group not stabilized the Ru–NO bond. These substitutions involving ligands in the trans position to NO stabilized the Ru–NO bond. Complexes with σ-donor/π-acceptor ligands compared to compounds with π-donor ligands in the cis or trans position to the NO group destabilized the Ru–NO bond. Charge distribution investigation realized from the Voronoi deformation density (VDD) method presents the Ru–NO bond more stabilized by negatively charged σ-donor, π-donor and σ-donor/π-acceptor ligands, in the cis or trans position to NO. These findings provide crucial information to the rational design of new NO storage–release systems with potential to deliver NO to desired targets in a controlled manner.
中文翻译:

配体的相对位置(顺式-反式)是否可以真正调节钌亚硝酰基络合物中NO的配位?
一氧化氮参与一系列生物过程。钌四胺络合物是控制NO可用性的模型结构。配体的影响对于确定Ru-NO键的稳定性至关重要。在本文中,不同性质和电荷,即,σ供体(NH的配体3和H - ),(ⅱ)π供体(H 2 O和NH 2 - ),和(iii)σ给体/π-受体(CO和CN - )相对于由相同的配体不仅在促进效果进行了评价顺位置向NO基团,而且在反式位置为NO。能量分解分析显示线性Ru–NO +和弯曲Ru–NO 0四氨合钌络合物中的键与NO基团的顺式或反式位置无关。总的来说,用一个π供体配体在NO的顺位取代一个π供体配体不能稳定Ru-NO键。这些涉及到NO反位配体的取代稳定了Ru-NO键。与在顺式或反式中具有π供体配体的化合物相比,具有σ供体/π受体配体的复合物NO基团的位置使Ru-NO键不稳定。通过Voronoi变形密度(VDD)方法实现的电荷分布研究表明,Ru-NO键通过带负电荷的σ-供体,π-供体和σ-供体/π-受体配体在NO的顺式或反式位置更稳定。这些发现为合理设计新的NO储存-释放系统提供了关键信息,有可能以受控方式将NO输送到所需的目标。
更新日期:2021-01-11
中文翻译:

配体的相对位置(顺式-反式)是否可以真正调节钌亚硝酰基络合物中NO的配位?
一氧化氮参与一系列生物过程。钌四胺络合物是控制NO可用性的模型结构。配体的影响对于确定Ru-NO键的稳定性至关重要。在本文中,不同性质和电荷,即,σ供体(NH的配体3和H - ),(ⅱ)π供体(H 2 O和NH 2 - ),和(iii)σ给体/π-受体(CO和CN - )相对于由相同的配体不仅在促进效果进行了评价顺位置向NO基团,而且在反式位置为NO。能量分解分析显示线性Ru–NO +和弯曲Ru–NO 0四氨合钌络合物中的键与NO基团的顺式或反式位置无关。总的来说,用一个π供体配体在NO的顺位取代一个π供体配体不能稳定Ru-NO键。这些涉及到NO反位配体的取代稳定了Ru-NO键。与在顺式或反式中具有π供体配体的化合物相比,具有σ供体/π受体配体的复合物NO基团的位置使Ru-NO键不稳定。通过Voronoi变形密度(VDD)方法实现的电荷分布研究表明,Ru-NO键通过带负电荷的σ-供体,π-供体和σ-供体/π-受体配体在NO的顺式或反式位置更稳定。这些发现为合理设计新的NO储存-释放系统提供了关键信息,有可能以受控方式将NO输送到所需的目标。











































 京公网安备 11010802027423号
京公网安备 11010802027423号