当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The role of 3-OH in the self-assembly of pharmaceutical cocrystals of dihydroflavonol with 4,4′-bipyridine
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2020-12-21 , DOI: 10.1039/d0nj04113k Lixin Liu 1, 2, 3, 4 , Moqi Liu 1, 2, 3, 4 , Yunan Zhang 1, 2, 3, 4 , Hemei Yin 1, 2, 3, 4 , Xin Su 1, 2, 3, 4 , Qiang Zhang 1, 2, 3, 4 , Yanru Feng 1, 2, 3, 4 , Yingxue Guo 1, 2, 3, 4 , Dongyu Zou 1, 2, 3, 4 , Yingli Liu 1, 2, 3, 4
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2020-12-21 , DOI: 10.1039/d0nj04113k Lixin Liu 1, 2, 3, 4 , Moqi Liu 1, 2, 3, 4 , Yunan Zhang 1, 2, 3, 4 , Hemei Yin 1, 2, 3, 4 , Xin Su 1, 2, 3, 4 , Qiang Zhang 1, 2, 3, 4 , Yanru Feng 1, 2, 3, 4 , Yingxue Guo 1, 2, 3, 4 , Dongyu Zou 1, 2, 3, 4 , Yingli Liu 1, 2, 3, 4
Affiliation
|
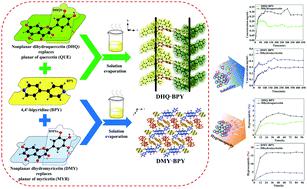
In order to further explore the role of hydroxyl in the synthesis of pharmaceutical cocrystals and with a view to improving physicochemical properties of flavonoids, two dihydroflavonols in which 3-OH is exposed and cross-conjugated system is destroyed (dihydroquercetin (DHQ) and dihydromyricetin (DMY)) were selected to combine with 4,4′-bipyridine (BPY) to explore the role of 3-OH of dihydroflavonol in synthetic processes of pharmaceutical cocrystals of dihydroflavonol. As a result, two new pharmaceutical cocrystals of dihydroflavonol with BPY (2(C15H12O7)·3(C10H8N2), DHQ·BPY); and (C15H12O8·2.5(C10H8N2), DMY·BPY) have been synthesized and characterized. Structure analysis revealed that in DHQ·BPY the intermolecular hydrogen bond formed by 3-OH of DHQ with BPY participated in the formation of motifs R66(44) to form the cocrystal with helical structure. In DMY·BPY, the intermolecular hydrogen bond formed by 3-OH of DMY with BPY connects adjacent motifs R44(38) to form the cocrystal with layered structure. The solubility of DHQ·BPY is lower than that of pure DHQ, possibly because the helical structure of DHQ·BPY is tighter than that of nonplanar DHQ, which limits the entry of water molecules. Compared with nonplanar DMY, DMY·BPY with layered structure is more likely to allow water molecules to enter, which leads to the solubility of DMY·BPY being higher than that of pure DMY. The hygroscopic stabilities of DHQ·BPY and DMY·BPY are better than that of their corresponding parent APIs. It is possible that in DHQ·BPY and DMY·BPY, most of the hydroxyls in the A, B and C rings of DHQ and DMY are involved in the formation of intermolecular hydrogen bonds, which means the hydroxyls of DHQ·BPY and DMY·BPY are difficult to combine with moisture. This work shows that 3-OH of nonplanar dihydroflavonol can form hydrogen bonds with BPY after the destruction of the cross-conjugated system, and then form cocrystals with helical structure and layered structure, thereby having an effect on the properties of APIs. This successful study implies that the role of 3-OH in the self-assembly process of flavonoid pharmaceutical cocrystals and improving the physicochemical properties of flavonoid APIs should not be neglected in the future.
中文翻译:

3-OH在二氢黄酮醇与4,4'-联吡啶的药物共晶体自组装中的作用
为了进一步探讨羟基在药物共晶体合成中的作用并改善类黄酮的理化性质,我们公开了两种暴露于3-OH且交叉共轭体系的二氢黄酮醇(二氢槲皮素(DHQ)和二氢杨梅素(选择DMY)与4,4'-联吡啶(BPY)结合,探讨二氢黄酮醇的3-OH在二氢黄酮醇药物共晶体的合成过程中的作用。结果,二氢黄酮醇与BPY(2(C 15 H 12 O 7)·3(C 10 H 8 N 2),DHQ·BPY)的两个新的药物共结晶;和(C 15 H 12 O 8 ·2.5(C已经合成并表征了10 H 8 N 2),DMY·BPY)。结构分析表明,在DHQ·BPY中,DHQ的3-OH与BPY形成的分子间氢键参与了基序R 6 6(44)的形成,形成了具有螺旋结构的共晶。在DMY·BPY中,由DMY的3-OH与BPY形成的分子间氢键连接相邻的基序R 4 4(38)形成具有层状结构的共晶体。DHQ·BPY的溶解度低于纯DHQ,可能是因为DHQ·BPY的螺旋结构比非平面DHQ的螺旋结构更紧密,从而限制了水分子的进入。与非平面DMY相比,具有分层结构的DMY·BPY更有可能使水分子进入,这导致DMY·BPY的溶解度高于纯DMY。DHQ·BPY和DMY·BPY的吸湿稳定性优于相应的母体API。在DHQ·BPY和DMY·BPY中,DHQ和DMY的A,B和C环中的大多数羟基可能参与分子间氢键的形成,这意味着DHQ·BPY和DMY·的羟基BPY难以与水分结合。这项工作表明,非平面二氢黄酮醇的3-OH可以在破坏交叉共轭体系后与BPY形成氢键,然后形成具有螺旋结构和层状结构的共晶体,从而对API的性能产生影响。这项成功的研究表明,3-OH在类黄酮药物共晶体的自组装过程中以及在改善类黄酮API的理化性质方面的作用在未来不应被忽略。
更新日期:2021-01-11
中文翻译:

3-OH在二氢黄酮醇与4,4'-联吡啶的药物共晶体自组装中的作用
为了进一步探讨羟基在药物共晶体合成中的作用并改善类黄酮的理化性质,我们公开了两种暴露于3-OH且交叉共轭体系的二氢黄酮醇(二氢槲皮素(DHQ)和二氢杨梅素(选择DMY)与4,4'-联吡啶(BPY)结合,探讨二氢黄酮醇的3-OH在二氢黄酮醇药物共晶体的合成过程中的作用。结果,二氢黄酮醇与BPY(2(C 15 H 12 O 7)·3(C 10 H 8 N 2),DHQ·BPY)的两个新的药物共结晶;和(C 15 H 12 O 8 ·2.5(C已经合成并表征了10 H 8 N 2),DMY·BPY)。结构分析表明,在DHQ·BPY中,DHQ的3-OH与BPY形成的分子间氢键参与了基序R 6 6(44)的形成,形成了具有螺旋结构的共晶。在DMY·BPY中,由DMY的3-OH与BPY形成的分子间氢键连接相邻的基序R 4 4(38)形成具有层状结构的共晶体。DHQ·BPY的溶解度低于纯DHQ,可能是因为DHQ·BPY的螺旋结构比非平面DHQ的螺旋结构更紧密,从而限制了水分子的进入。与非平面DMY相比,具有分层结构的DMY·BPY更有可能使水分子进入,这导致DMY·BPY的溶解度高于纯DMY。DHQ·BPY和DMY·BPY的吸湿稳定性优于相应的母体API。在DHQ·BPY和DMY·BPY中,DHQ和DMY的A,B和C环中的大多数羟基可能参与分子间氢键的形成,这意味着DHQ·BPY和DMY·的羟基BPY难以与水分结合。这项工作表明,非平面二氢黄酮醇的3-OH可以在破坏交叉共轭体系后与BPY形成氢键,然后形成具有螺旋结构和层状结构的共晶体,从而对API的性能产生影响。这项成功的研究表明,3-OH在类黄酮药物共晶体的自组装过程中以及在改善类黄酮API的理化性质方面的作用在未来不应被忽略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号