当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of C3-alkenylated 2,3,4-trisubstituted pyrrole derivatives through cyclization of methylene isocyanides and ene–yne–ketones
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2020-12-21 , DOI: 10.1039/d0nj05253a Shasha Li 1, 2, 3, 4 , Gongruixue Zeng 4, 5, 6, 7 , Xiaoqi Xing 4, 5, 6, 7 , Zhiheng Yang 1, 2, 3, 4 , Feiyun Ma 1, 2, 3, 4 , Boxia Li 1, 2, 3, 4 , Weiyan Cheng 1, 2, 3, 4 , Jiankang Zhang 4, 5, 6, 7 , Ruoyu He 4, 8, 9, 10
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2020-12-21 , DOI: 10.1039/d0nj05253a Shasha Li 1, 2, 3, 4 , Gongruixue Zeng 4, 5, 6, 7 , Xiaoqi Xing 4, 5, 6, 7 , Zhiheng Yang 1, 2, 3, 4 , Feiyun Ma 1, 2, 3, 4 , Boxia Li 1, 2, 3, 4 , Weiyan Cheng 1, 2, 3, 4 , Jiankang Zhang 4, 5, 6, 7 , Ruoyu He 4, 8, 9, 10
Affiliation
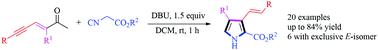
|
A mild, transition-metal-free and facile C3-alkenylated 2,3,4-trisubstituted pyrrole cyclization of methylene isocyanides with ene–yne–ketones in moderate to good yields was explored. The E-alkenylated products were isolated in moderate to exclusive selectivity in most cases. The investigated compounds in this work are expected to open up for use as potential medicinal agents or precursors for post-modification.
中文翻译:

通过亚甲基异氰酸酯和烯-炔-酮的环化反应合成C3-烯基化的2,3,4-三取代的吡咯衍生物
探索了温和,无过渡金属且轻度的C3-烯基化的2,3,4-三取代吡咯亚甲基异氰酸酯与烯-炔-酮的环化反应,产率中等至良好。在大多数情况下,以中等至排他选择性分离出E-烯基化产物。预期这项工作中所研究的化合物将开放用作潜在的后修饰药物或前体。
更新日期:2021-01-11
中文翻译:

通过亚甲基异氰酸酯和烯-炔-酮的环化反应合成C3-烯基化的2,3,4-三取代的吡咯衍生物
探索了温和,无过渡金属且轻度的C3-烯基化的2,3,4-三取代吡咯亚甲基异氰酸酯与烯-炔-酮的环化反应,产率中等至良好。在大多数情况下,以中等至排他选择性分离出E-烯基化产物。预期这项工作中所研究的化合物将开放用作潜在的后修饰药物或前体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号