当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ifenprodil Stereoisomers: Synthesis, Absolute Configuration, and Correlation with Biological Activity
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-01-10 , DOI: 10.1021/acs.jmedchem.0c01912 Elena Bechthold 1, 2 , Julian A. Schreiber 2, 3 , Kirstin Lehmkuhl 2 , Bastian Frehland 2 , Dirk Schepmann 2 , Freddy A. Bernal 4 , Constantin Daniliuc 5 , Inés Álvarez 6 , Cristina Val Garcia 7 , Thomas J. Schmidt 4 , Guiscard Seebohm 1, 3, 7 , Bernhard Wünsch 1, 2
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-01-10 , DOI: 10.1021/acs.jmedchem.0c01912 Elena Bechthold 1, 2 , Julian A. Schreiber 2, 3 , Kirstin Lehmkuhl 2 , Bastian Frehland 2 , Dirk Schepmann 2 , Freddy A. Bernal 4 , Constantin Daniliuc 5 , Inés Álvarez 6 , Cristina Val Garcia 7 , Thomas J. Schmidt 4 , Guiscard Seebohm 1, 3, 7 , Bernhard Wünsch 1, 2
Affiliation
|
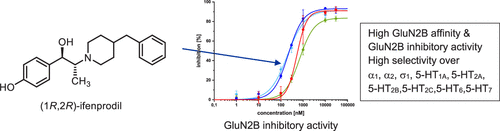
Ifenprodil (1) is a potent GluN2B-selective N-methyl-d-aspartate (NMDA) receptor antagonist that is used as a cerebral vasodilator and has been examined in clinical trials for the treatment of drug addiction, idiopathic pulmonary fibrosis, and COVID-19. To correlate biological data with configuration, all four ifenprodil stereoisomers were prepared by diastereoselective reduction and subsequent separation of enantiomers by chiral HPLC. The absolute configuration of ifenprodil stereoisomers was determined by X-ray crystal structure analysis of (1R,2S)-1a and (1S,2S)-1d. GluN2B affinity, ion channel inhibitory activity, and selectivity over α, σ, and 5-HT receptors were evaluated. (1R,2R)-Ifenprodil ((1R,2R)-1c) showed the highest affinity toward GluN2B-NMDA receptors (Ki = 5.8 nM) and high inhibition of ion flux in two-electrode voltage clamp experiments (IC50 = 223 nM). Whereas the configuration did not influence considerably the GluN2B-NMDA receptor binding, (1R)-configuration is crucial for elevated inhibitory activity. (1R,2R)-Configured ifenprodil (1R,2R)-1c exhibited high selectivity for GluN2B-NMDA receptors over adrenergic, serotonergic, and σ1 receptors.
中文翻译:

Ifenprodil立体异构体:合成,绝对构型以及与生物活性的相关性
艾芬地尔(1)是一种强效GluN2B选择性Ñ甲基d被用作血管舒张剂脑和临床试验已经研究了用于药物成瘾,特发性肺纤维化,和COVID-治疗天冬氨酸(NMDA)受体拮抗剂19 为了使生物学数据与构型相关联,通过非对映选择性还原并随后通过手性HPLC分离对映异构体来制备所有四种艾芬地尔立体异构体。的立体异构体艾芬地尔的绝对构型由(1 X射线晶体结构分析来确定- [R,2小号- )1A和(1小号,2小号) - 1D。评估了GluN2B亲和力,离子通道抑制活性以及对α,σ和5-HT受体的选择性。(1 - [R,2 - [R)-Ifenprodil((1 - [R,2 - [R )- 1C),发现其朝GluN2B-NMDA受体具有最高的亲和力(ķ我= 5.8纳米)和高抑制离子通量的在双电极电压钳实验( IC 50 = 223 nM)。尽管该构型不会显着影响GluN2B-NMDA受体的结合,但是(1R)-构型对于提高抑制活性至关重要。(1 R,2 R)-配置的艾芬地尔(1 R,2 R)-1c表现出对GluN2B-NMDA受体过度肾上腺素,血清素能高选择性,和σ 1个受体。
更新日期:2021-01-28
中文翻译:

Ifenprodil立体异构体:合成,绝对构型以及与生物活性的相关性
艾芬地尔(1)是一种强效GluN2B选择性Ñ甲基d被用作血管舒张剂脑和临床试验已经研究了用于药物成瘾,特发性肺纤维化,和COVID-治疗天冬氨酸(NMDA)受体拮抗剂19 为了使生物学数据与构型相关联,通过非对映选择性还原并随后通过手性HPLC分离对映异构体来制备所有四种艾芬地尔立体异构体。的立体异构体艾芬地尔的绝对构型由(1 X射线晶体结构分析来确定- [R,2小号- )1A和(1小号,2小号) - 1D。评估了GluN2B亲和力,离子通道抑制活性以及对α,σ和5-HT受体的选择性。(1 - [R,2 - [R)-Ifenprodil((1 - [R,2 - [R )- 1C),发现其朝GluN2B-NMDA受体具有最高的亲和力(ķ我= 5.8纳米)和高抑制离子通量的在双电极电压钳实验( IC 50 = 223 nM)。尽管该构型不会显着影响GluN2B-NMDA受体的结合,但是(1R)-构型对于提高抑制活性至关重要。(1 R,2 R)-配置的艾芬地尔(1 R,2 R)-1c表现出对GluN2B-NMDA受体过度肾上腺素,血清素能高选择性,和σ 1个受体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号