当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Urea nitrate–catalyzed C‐N and C‐S bond formation: A mechanochemical approach for 5‐chloro‐2‐arylbenzo[d]thiazole derivatives
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2021-01-11 , DOI: 10.1002/jhet.4224 Ayushi Sethiya 1 , Nusrat Sahiba 1 , Jay Soni 1 , Shikha Agarwal 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2021-01-11 , DOI: 10.1002/jhet.4224 Ayushi Sethiya 1 , Nusrat Sahiba 1 , Jay Soni 1 , Shikha Agarwal 1
Affiliation
|
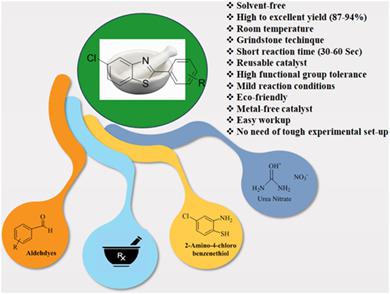
A series of substituted 5‐chloro‐2‐arylbenzo[d]thiazoles were synthesized using 4‐chloro‐2‐aminothiophenol and aromatic aldehydes in the presence of urea nitrate as a catalyst using the mechanochemical grindstone technique. This protocol was effectively carried out under metal‐free conditions at room temperature, and the desired products were obtained in high to excellent yields in short reaction time (30–60 s). The structure of all the synthesized derivatives was confirmed by spectral characterization. The designed protocol has several benefits like eco‐friendly, solvent‐free, high yields, easy workup, and recyclability of catalyst. The catalyst was reusable at least four times without significant loss of activity. The good functional group tolerance with a series of derivatives has been demonstrated.
中文翻译:

硝酸尿素催化的C-N和C-S键形成:5-氯-2-芳基苯并[d]噻唑衍生物的机械化学方法
使用4-氯-2-氨基苯硫酚和芳香醛在硝酸尿素作为催化剂的情况下,使用机械化学砂轮技术合成了一系列取代的5-氯-2-芳基苯并[d]噻唑。该方案有效地在室温下在无金属条件下进行,并且可以在较短的反应时间(30-60 s)内以高收率或优异的收率获得所需的产物。所有合成的衍生物的结构通过光谱表征证实。设计的方案具有多个优点,例如环保,无溶剂,高收率,易于后处理和催化剂的可回收性。催化剂可重复使用至少四次,而活性没有明显损失。已经证明了一系列衍生物具有良好的官能团耐受性。
更新日期:2021-03-02
中文翻译:

硝酸尿素催化的C-N和C-S键形成:5-氯-2-芳基苯并[d]噻唑衍生物的机械化学方法
使用4-氯-2-氨基苯硫酚和芳香醛在硝酸尿素作为催化剂的情况下,使用机械化学砂轮技术合成了一系列取代的5-氯-2-芳基苯并[d]噻唑。该方案有效地在室温下在无金属条件下进行,并且可以在较短的反应时间(30-60 s)内以高收率或优异的收率获得所需的产物。所有合成的衍生物的结构通过光谱表征证实。设计的方案具有多个优点,例如环保,无溶剂,高收率,易于后处理和催化剂的可回收性。催化剂可重复使用至少四次,而活性没有明显损失。已经证明了一系列衍生物具有良好的官能团耐受性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号