Synthetic Metals ( IF 4.0 ) Pub Date : 2021-01-11 , DOI: 10.1016/j.synthmet.2020.116689 Paweł Nitschke , Bożena Jarząbek , Marharyta Vasylieva , Damian Honisz , Jan Grzegorz Małecki , Marta Musioł , Henryk Janeczek , Paweł Chaber
|
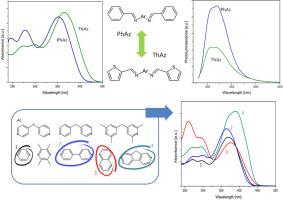
A correlation between a chemical structure of π-conjugated azomethines and their physicochemical properties has been studied, towards their optoelectronic applications. Eight various homo-aryl moieties have been coupled with either phenylene or thiophene groups, via imine bond, resulting in 15 (9 novel) azomethines. Differential scanning calorimetry (DSC), thermogravimetric analysis (TG), UV-Vis absorption and photoluminescence spectroscopy, and cyclic voltammetry techniques have been used to investigate their thermal, optical and electrochemical properties. Moreover, these results have been supported by the density functional theory (DFT) calculations. Introduction of thiophene rings, into the structure of studied azomethines, proved to increase resistance towards hydrolysis, their thermal stability and reduction potential, together with an enhancement of their π-delocalization. Simultaneously, the presence of thiophene rings in azomethine structures has decreased their emissive properties, compared to their completely homo-aryl analogues. The highest thermal stability and the most enhanced conjugation has been observed for azomethines consisting of such central units moieties as fluorene, naphtalene and biphenylene. Surprisingly, the presence of conjugation breaking ether or methylene bridge provides interesting photoluminescence properties, when coupled with thiophene. Finally, the substitution with side methyl groups proved to induce a reduction of oxidation potential and an increase of melting temperatures. Thiophene, fluorene, naphtalene and biphenylene groups, due to enhancement of thermal stability and extension of absorption range, appear to be good building units for novel materials for photovoltaic structures, while the most promising properties in the context of application in emissive devices have been observed for phenylene-coupled compounds with such groups as phenylene or biphenylene.
中文翻译:

化学结构对共轭甲亚胺的热,光学和电化学性质的影响
针对它们的光电应用,已经研究了π共轭偶氮甲碱的化学结构与其物理化学性质之间的相关性。已通过亚胺键将八个不同的高芳基部分与亚苯基或噻吩基偶合,产生15个(9个新的)偶氮甲胺。差示扫描量热法(DSC),热重分析(TG),UV-Vis吸收和光致发光光谱以及循环伏安技术已用于研究其热,光学和电化学性质。而且,这些结果得到了密度泛函理论(DFT)计算的支持。在已研究的偶氮甲亚胺的结构中引入噻吩环可提高耐水解性,热稳定性和还原电位,以及它们的π离域的增强。同时,相比于它们的完全同芳基类似物,在偶氮甲碱结构中噻吩环的存在降低了它们的发射性质。对于由诸如芴,萘和联苯之类的中心单元部分组成的偶氮甲胺,已经观察到最高的热稳定性和最强的共轭。出人意料的是,当与噻吩偶联时,共轭断裂的醚或亚甲基桥的存在提供了令人感兴趣的光致发光性质。最后,证明用侧甲基取代可以引起氧化电位降低和熔融温度升高。噻吩,芴,萘和联苯基团由于增强了热稳定性并扩展了吸收范围,











































 京公网安备 11010802027423号
京公网安备 11010802027423号