当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ubiquitin‐independent proteasomal degradation of Spindlin‐1 by the E3 ligase HACE1 contributes to cell‐cell adhesion
FEBS Letters ( IF 3.0 ) Pub Date : 2021-02-02 , DOI: 10.1002/1873-3468.14031 Vivek Reddy Palicharla 1 , Devanshi Gupta 1, 2 , Debjani Bhattacharya 1 , Subbareddy Maddika 1
FEBS Letters ( IF 3.0 ) Pub Date : 2021-02-02 , DOI: 10.1002/1873-3468.14031 Vivek Reddy Palicharla 1 , Devanshi Gupta 1, 2 , Debjani Bhattacharya 1 , Subbareddy Maddika 1
Affiliation
|
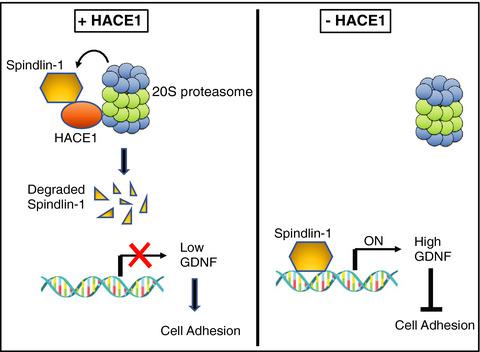
HECT-E3 ligases play an essential role in catalyzing the transfer of ubiquitin to protein substrates. The non-catalytic roles of HECT-E3 ligases in cells are unknown. Here, we report that a HECT-E3 ligase, HACE1, functions as an adaptor independent of its E3 ligase activity. We identified Spindlin-1, a histone reader, as a new HACE1-associated protein. Interestingly, we found that HACE1 promotes Spindlin-1 degradation via the proteasome in a ubiquitination- independent manner. Functionally, we demonstrated that loss of HACE1 results in weak cell-cell adhesion due to Spindlin-1-mediated accumulation of GDNF, a negative regulator of cell adhesion. Together, our data suggest that HACE1 acts as a molecular adaptor and plays an important non-catalytic role in presenting selected substrates directly to the proteasome for degradation.
中文翻译:

E3连接酶HACE1对Spindlin-1的非泛素依赖性蛋白酶体降解有助于细胞-细胞粘附
HECT-E3 连接酶在催化泛素向蛋白质底物转移方面发挥着重要作用。HECT-E3 连接酶在细胞中的非催化作用尚不清楚。在这里,我们报告了 HECT-E3 连接酶 HACE1 作为独立于其 E3 连接酶活性的适配器。我们将组蛋白读取器 Spindlin-1 鉴定为一种新的 HACE1 相关蛋白。有趣的是,我们发现 HACE1 通过蛋白酶体以不依赖泛素化的方式促进 Spindlin-1 降解。在功能上,我们证明了由于 Spindlin-1 介导的 GDNF(细胞粘附的负调节因子)的积累,HACE1 的缺失导致细胞间粘附较弱。总之,我们的数据表明 HACE1 作为分子接头并在将选定的底物直接呈递给蛋白酶体进行降解方面发挥重要的非催化作用。
更新日期:2021-02-02
中文翻译:

E3连接酶HACE1对Spindlin-1的非泛素依赖性蛋白酶体降解有助于细胞-细胞粘附
HECT-E3 连接酶在催化泛素向蛋白质底物转移方面发挥着重要作用。HECT-E3 连接酶在细胞中的非催化作用尚不清楚。在这里,我们报告了 HECT-E3 连接酶 HACE1 作为独立于其 E3 连接酶活性的适配器。我们将组蛋白读取器 Spindlin-1 鉴定为一种新的 HACE1 相关蛋白。有趣的是,我们发现 HACE1 通过蛋白酶体以不依赖泛素化的方式促进 Spindlin-1 降解。在功能上,我们证明了由于 Spindlin-1 介导的 GDNF(细胞粘附的负调节因子)的积累,HACE1 的缺失导致细胞间粘附较弱。总之,我们的数据表明 HACE1 作为分子接头并在将选定的底物直接呈递给蛋白酶体进行降解方面发挥重要的非催化作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号