当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of new Schiff bases; Investigation of their in situ catalytic activity for Suzuki C?C coupling reactions and antioxidant activities
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2021-01-08 , DOI: 10.1002/jccs.202000433 Özgür Yılmaz 1
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2021-01-08 , DOI: 10.1002/jccs.202000433 Özgür Yılmaz 1
Affiliation
|
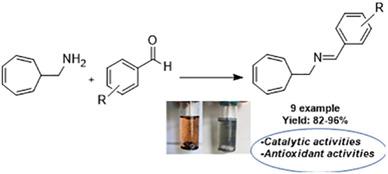
New series of Schiff bases have been synthesized from the reaction between cyclohepta-2,4,6-trien-1-ylmethanamine and different aldehydes, and characterized via using 1H NMR, 13C NMR, FTIR spectroscopy, and GC–MS. After the successful synthesis, the in situ catalytic activity of all Schiff bases have been examined for the Suzuki CC cross-coupling reactions using phenylboronic acid, aryl bromides, and PdCl2 as a catalyst. Before starting these investigations, reaction conditions were optimized using different bases and solvents. At the end of these reactions, the best efficiency was obtained in Et3N and EtOH. In addition to catalytic investigations, antioxidant activities of all synthesized Schiff bases were examined using DPPH and Iron (Fe2+) chelation methods, and IC50 values were calculated. While many molecules show various amounts of antioxidant activity, especially molecules 8e and 8g showed the best activity compared to butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA), which were used as positive controls, in DPPH and Iron chelating methods, respectively.
中文翻译:

合成新的席夫碱;它们对铃木C ?的原位催化活性的研究 C偶联反应和抗氧化活性
通过环庚2,4,6-三烯-1-基甲胺与不同醛的反应合成了一系列新的席夫碱,并通过1 H NMR,13 C NMR,FTIR光谱和GC-MS对其进行了表征。成功合成后,已使用苯基硼酸,芳基溴化物和PdCl 2作为催化剂,检查了所有Schiff碱在SuzukiCC交叉偶联反应中的原位催化活性。在开始这些研究之前,使用不同的碱和溶剂优化反应条件。在这些反应结束时,在Et 3中获得了最佳效率N和EtOH。除了进行催化研究外,还使用DPPH和铁(Fe 2+)螯合方法检查了所有合成的席夫碱的抗氧化活性,并计算了IC 50值。尽管许多分子显示出不同程度的抗氧化活性,但与分别用作DPPH和铁螯合方法的阳性对照的丁基化羟基甲苯(BHT)和丁基化羟基茴香醚(BHA)相比,分子8e和8g表现出最佳的活性。
更新日期:2021-01-08
中文翻译:

合成新的席夫碱;它们对铃木C ?的原位催化活性的研究 C偶联反应和抗氧化活性
通过环庚2,4,6-三烯-1-基甲胺与不同醛的反应合成了一系列新的席夫碱,并通过1 H NMR,13 C NMR,FTIR光谱和GC-MS对其进行了表征。成功合成后,已使用苯基硼酸,芳基溴化物和PdCl 2作为催化剂,检查了所有Schiff碱在SuzukiCC交叉偶联反应中的原位催化活性。在开始这些研究之前,使用不同的碱和溶剂优化反应条件。在这些反应结束时,在Et 3中获得了最佳效率N和EtOH。除了进行催化研究外,还使用DPPH和铁(Fe 2+)螯合方法检查了所有合成的席夫碱的抗氧化活性,并计算了IC 50值。尽管许多分子显示出不同程度的抗氧化活性,但与分别用作DPPH和铁螯合方法的阳性对照的丁基化羟基甲苯(BHT)和丁基化羟基茴香醚(BHA)相比,分子8e和8g表现出最佳的活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号